Amide Bond Formation in Cyclization
Traditional Amide Cyclization
Many naturally active peptides are cyclized head-to-tail, these peptides are more resistant to hydrolysis since the absence of N- and C-terminus. Traditional coupling chemistry is used to cyclize the liner peptide precursor by amide bond formation. However, conventional head-to-tail amide formation is non-trivial, especially for peptides shorter than seven residues.
There are three main peptide coupling reagents for amide formation: carbodiimides, phosphonium reagents, and aminium−/uronium–iminium reagents. The careful selection of coupling reagents and additives can reduce epimerization in cyclic synthesis. Amide bond formation is not chemoselective, the in-solution cyclization require sidechain-protection. Furthermore, the amide forming on solid support and pseudo-dilution effect can reduce inermolecular reactions.
Sulfur Mediated Amide Formation
Chemo-selective reactions are the prevailing strategy for the amide synthesis of head-to-tail cyclized peptides. A multitude of synthesis strategies employ the S-to-N transfer in ligation reactions.
Native Chemical Ligation
Native chemical ligation (NCL) is a mild and site-selective amide-formation reaction for peptide cycliztion, the N-terminal cysteine reacts with the C-terminal thioester in neutral and aqueous solution.
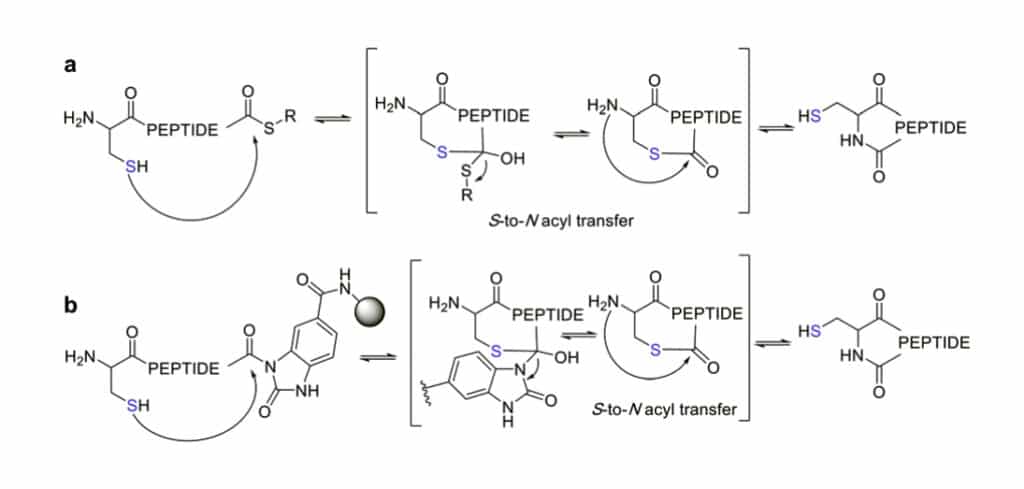
The excellent chemo-selectivity of NCL is generated by the poor nucleophilicity of other sidechains at pH7. The Thiols like PhSH or BnSH are nucleophilic catalysts to enable inter-molecular trans-thioesterification, low epimerization and no oligomerization are observed even at high concentrations.
NCL is a powerful method for chemoselective cyclization of head-to-tail peptide, there are still some limitations, like cysteine in peptide sequence. Qyaobio can accomplish the installation of thioester by different methods in SPPS: desulfurisation, thiol-containing auxiliary groups, cysteine surrogates.
Thiazolidine Cyclization
1,2-aminothiol of an N-terminal cysteine can condense with an aldehyde to form a thiazolidine ring. This chemoselective reaction incorporates the aldehyde as an oxidized C-terminal glycolaldehyde ester, in order to achieve the cycled peptide by a ring-contraction mechanism in a tricyclic intramolecular rearrangement.
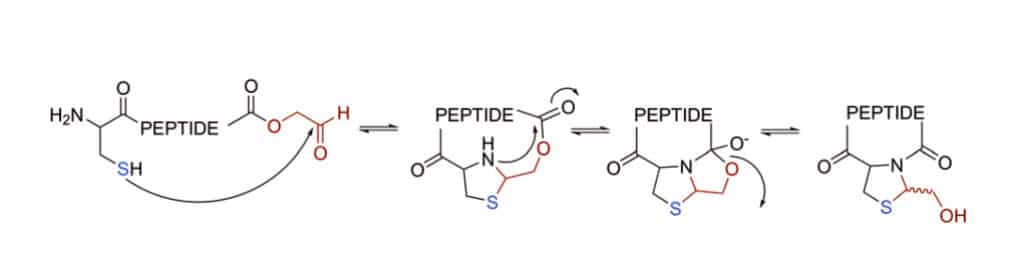
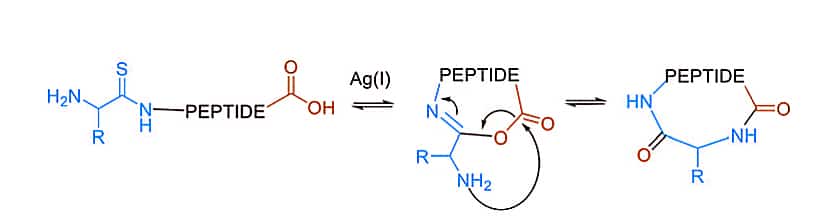
AgI Mediated Cyclization
AgI, as a contemporaty method, can promote the head-to-tail ligation of a C-terminal carboxylic acid and an N-terminal thioamide. Ag can activate the N-terminal thioamide chemoselectively, and bring it to the C-terminal carboxylate in proximity. After the extrusion of Ag2S and procedure of acyl transfer, the isoimide intermediate is formed as a tracelss macro-cyclization.
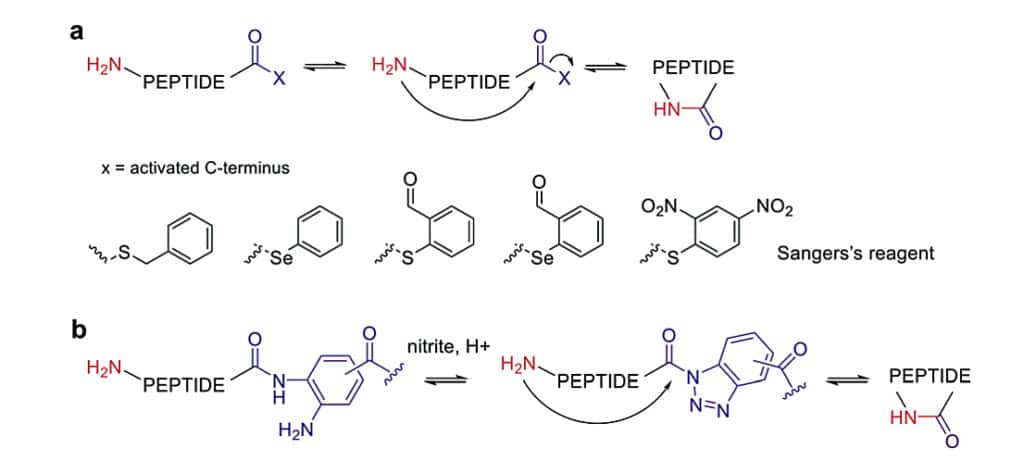
Aminolysis Mediated Cyclization
The aminolysis of C-terminal thioesters in an aqueous solution imidazole can be applied for peptide macro-cyclizations. This peptide cyclization is achieved in mild acidic conditions with additions of HOAt and HOBt. This aminolysis strategy has wide applicability to any peptide sequence without specific amino acid requirement, like cysteine.
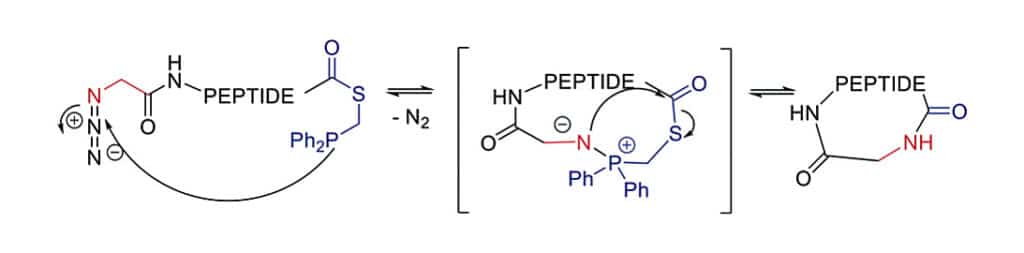
Staudinger Ligation Reaction
The Staudinger ligation is a chemoselective method for traceless head-to-tail cyclization of peptides. The C-terminal phosphino-thioester reacts with the N-terminal azide to create a cyclic iminophosphorane, then collapses to an amide bond by elimination of the thiophosphorane.
Amine-Reactive Stapling
Peptide stapling is introduced to react with the highly nucleophilic thiolate of cysteines, then adapted to react with amine sidechains subsequently. This stapling cyclzation method applies nucleophilic aromatic substitution and palladium-catalyzed arylation chemistry.
Functional Groups Amide Formation
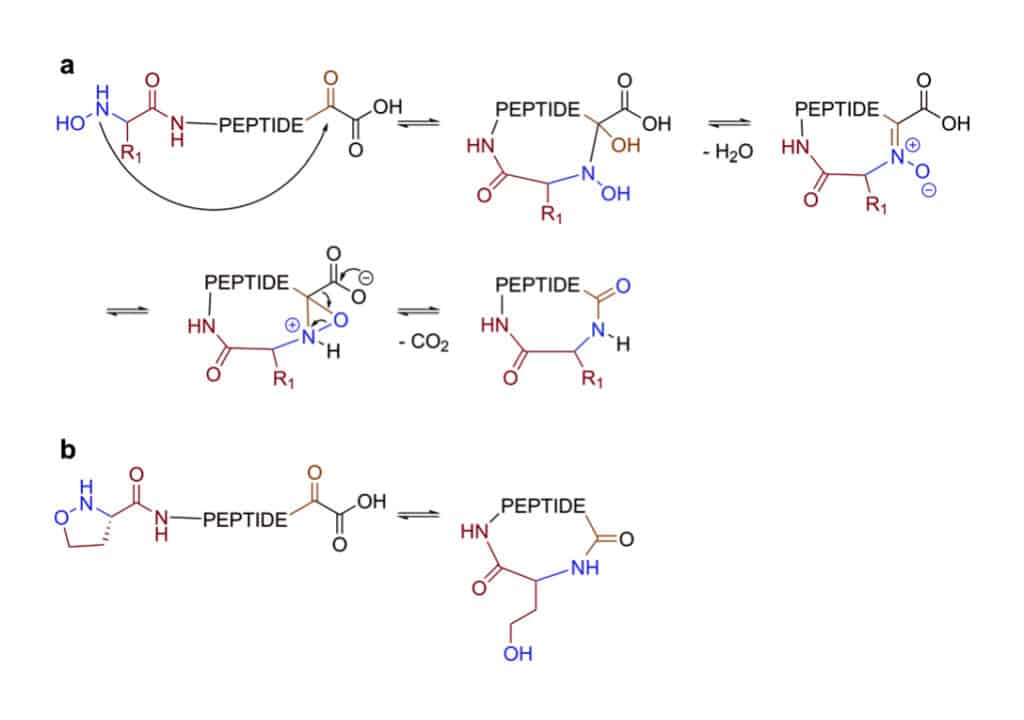
Ketoacid-Hydroxylamine (KAHA) Ligation
ketoacid-hydroxylamine (KAHA) ligation is a reaction between a C-terminal ketoacid and the N-terminal hydroxylamine of peptides. This ligation can yield macro-cyclic peptide from unprotected linear peptides in mild conditions with polar protic and aprotic solvents. However, the KAHA ligation has drawbacks of slow reaction, high epimerization rate, instability of hydroxylamines.
Ser/Thr Ligation
Ser/Thr ligation is developed for cyclic synthesis of peptide fragments containing a serine or threonine. This ligation technology is extended to backbone cyclization of tetrapeptides with N-terminal serine or threonine and C-terminal salicylaldehyde ester. The cyclic peptides in this ligation method have no epimerization.
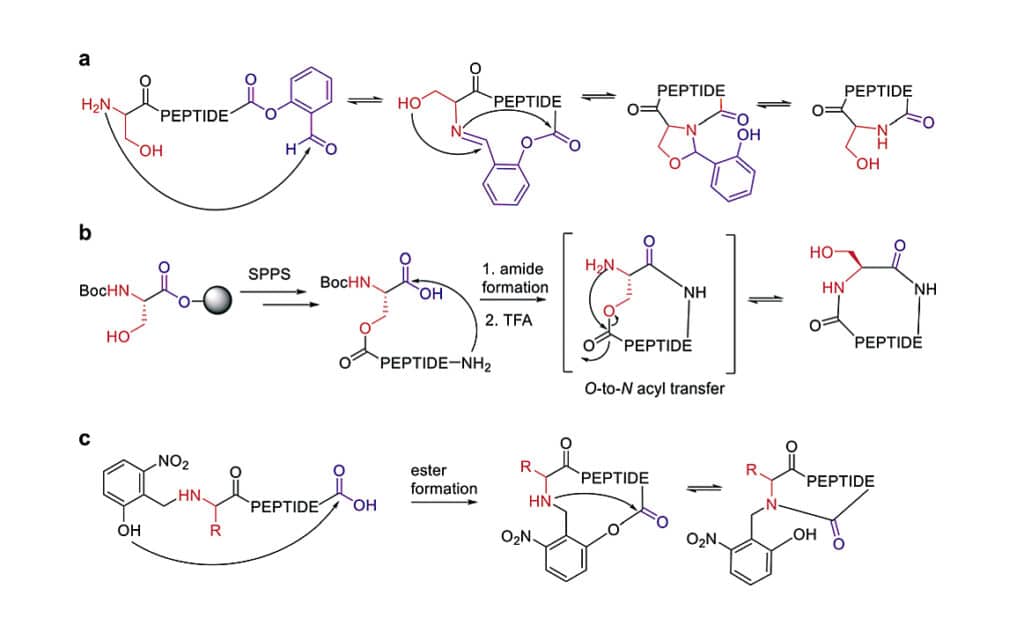
Macrocyclization of the depsipeptide is achieved by the O-to-N migration. This strategy still relies on the conventional head-to-tail cyclization, it can achieve the difficultly cyclic synthesis of penta or hexapeaptide.
The auxiliary groups are another application strategy for difficult sequence cyclization, the O-to-N acyl migration can generate the lactam, then the auxiliary group is released by exploiting its photo lability.
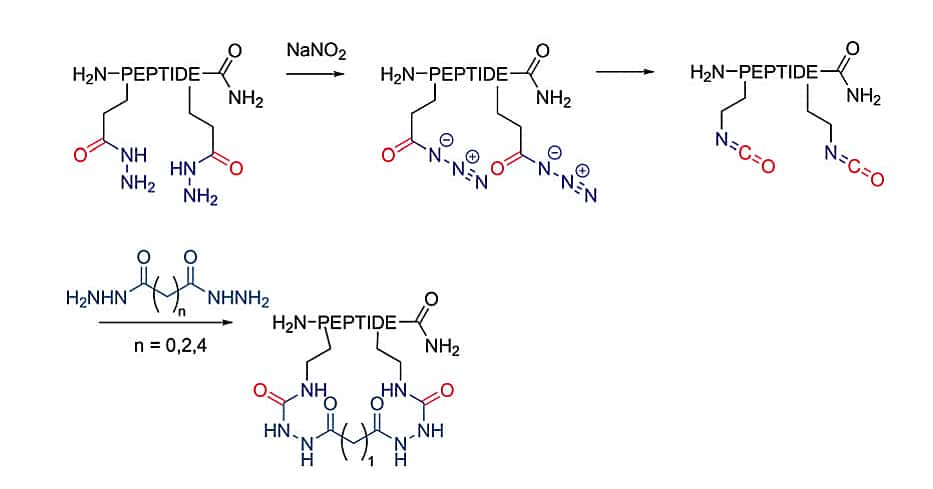
Isocyanates Formation
The isocyanate is synthesized in situ to react with hydrazides to yield semicarbazides, this is analogous to amine bond formation. It is a more robust cyclization for smaller ring sizes.
Imines Macrocyclization Chemistry
On-resin Imines Strategy
Imine forming chemistry develops the new macrocyclization between the aldehyde and the N-terminal primary amine. There are two different aldehyde installation methods in solid-phase approaches.
- Install the aldehyde on aspartate sidechain by reaction with amino acetaldehyde dimethyl acetal.
- Couple the α-amino aldehyde on a tyrosine-glycine resin.
In the second strategy, the C-terminal aldehyde is accessible from the resin cleavages.
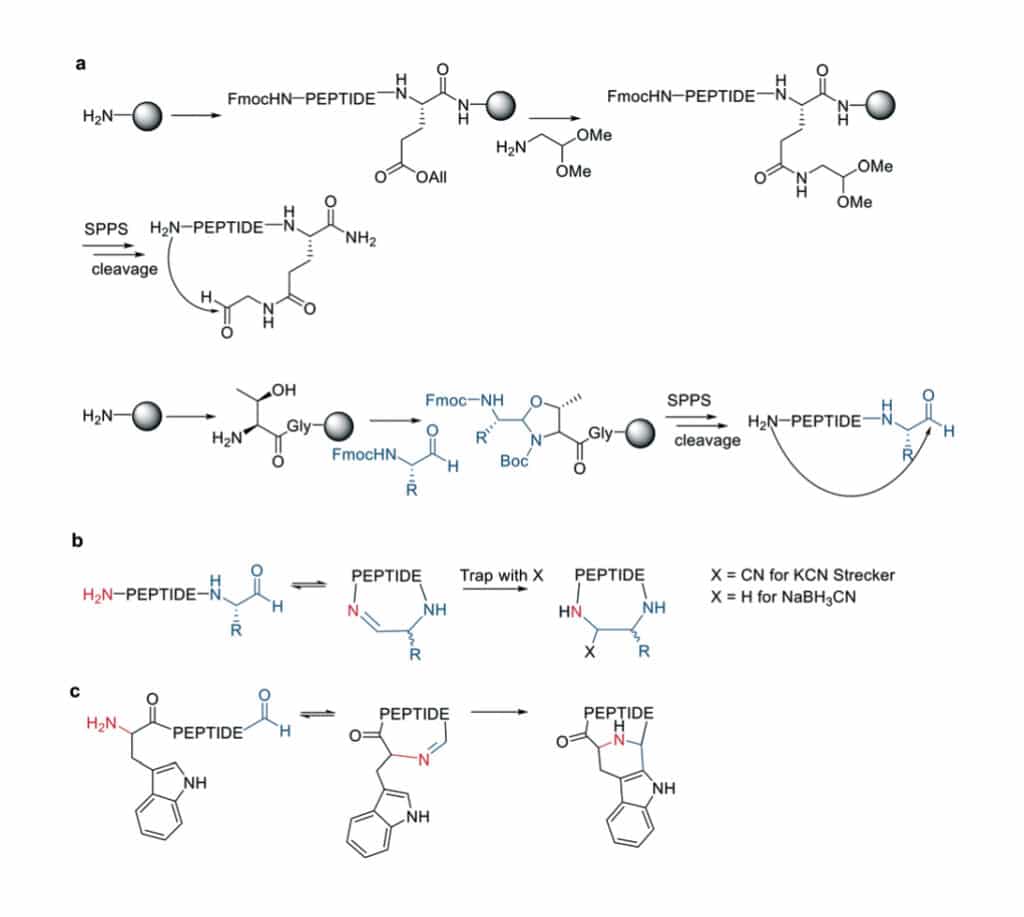
In addition, imines are also trapped as amines by reductive amination with NaBH3CN in aqueous NaOAc buffer.
Pictet-Spengler macro-cyclization indicates the intramolecular imine traps with aromatic rings like indoles and imidazoles.
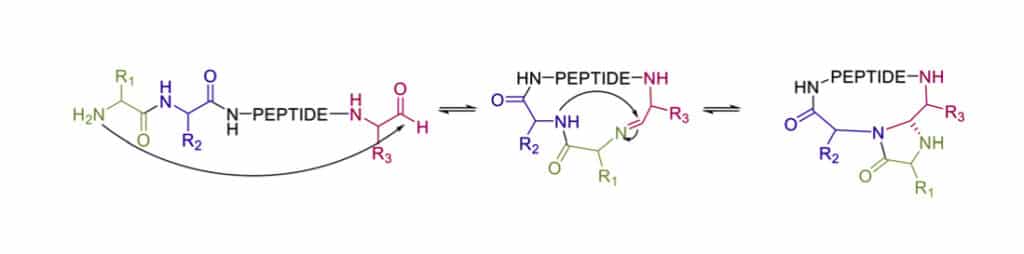
4-imidazolidinone Cyclization
After imine formation, the nearby nitrogen will attach the imine group to generate a stable 4-imidazolidinone. Since the high chemo-selectivity of intramolecular reaction, this cyclization can react at high concentration without oligomerization increase. Moreover, the 4 imidazolidinone is a turn-inducing element, it can increase intra-moleuclar hydrogen bonds, conformational rigidity, enzymatic stability.
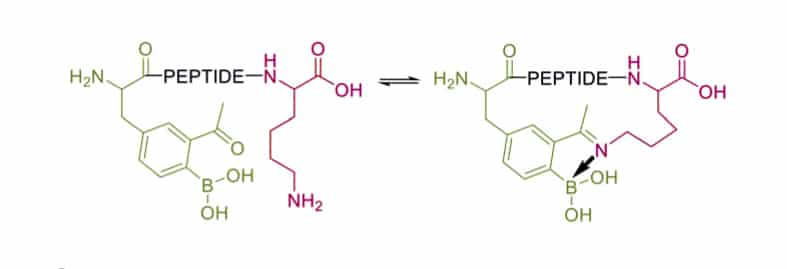
Iminoboronate Formation
Iminoboronate is another strategy to trap the imine, the introduction of boronic acid in peptides allow the rapid and spontaneous cyclization in physiologic conditions. Interestingly, this cyclization is reversed rapidly in response to acids, oxidation, and α-nucleophiles (hydrazine and amino alcohols).
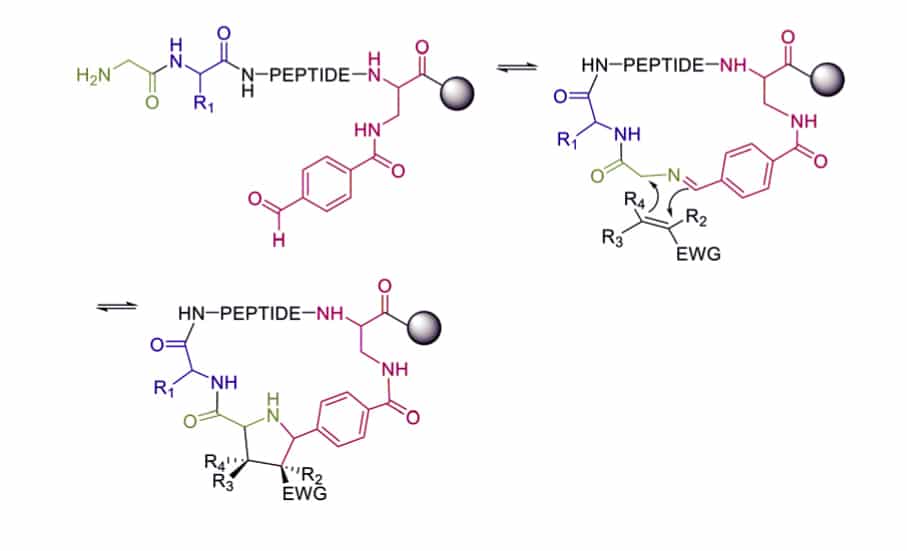
Dipolar Cycloaddition
The 1,3-dipolar cycloaddition can trap the imine, and generate fused and spiro-ring systems. Which is frequent in pharmacologically active natural products. The imine is synthesized on solid support between 4-carboxybenzaldehyde and the primary amine of N-terminal glycines.
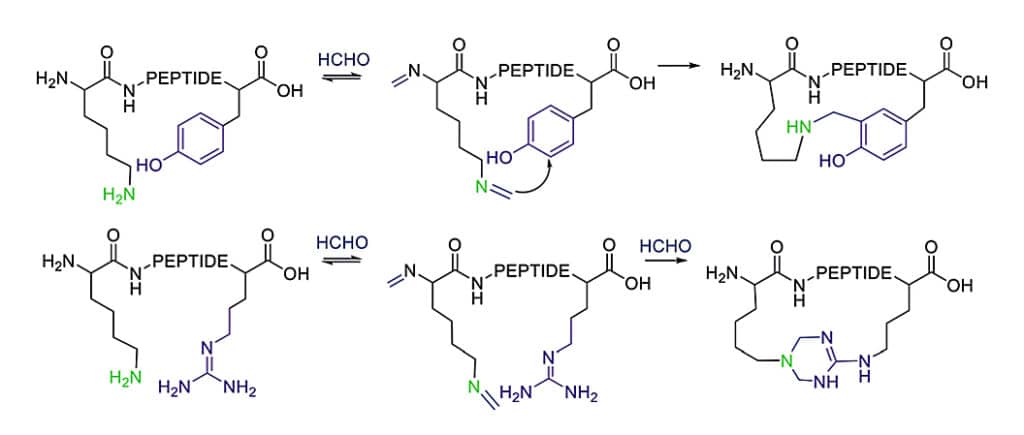
Cooperative Macrocyclization
Native peptides can react with formaldehyde to generate the imine with the ε-amino group of Lys, the imine can cross-link to nearby tyrosine or arginine residues.
Oximes Macrocyclization Chemistry
Oximes generate the most stable cyclization in dynamic covalent chemistry approaches with Schiff bases (imines, hydrazones, and oximes). The oxime formation in side-chain cyclization is achieved by noncanonical amino acids with a 1,2-aminoalcohol, which is oxidized to an aldehyde by NaIO4. The oxime formation is favored in thermodynamic but kinetically slow at neutral pH. Therefore, it is accelerated by acidic conditions or nucleophilic catalysts. In addition, oxime formation create two isomers: E and Z oximes.
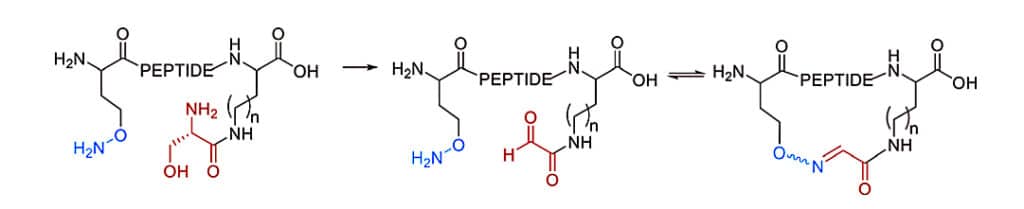
The furanylalanine in noncanonical amino acid also can be oxidized by N-bromosuccinimide (NBS) to form the ketoenal, then the ketoenal react with nucleophilic sidechains (Lys) to cyclize. This reaction is irreversible once imine trapping by reduction with NaCNBH3.