AMPs Introduction
Antimicrobial peptides are class of small peptides with widely natural exist, AMPs are the important part of the innate immune system. Antimicrobial peptides have a broad-spectrum inhibitory effect against bacteria, fungi, parasites and viruses. Antimicrobial peptides have an excellent application prospect in medicine, food, animal husbandry, agriculture, and aquaculture.
Antimicrobial peptides contain diverse molecules structure group, these peptides are in all virtual organisms. Most antimicrobial peptides contain 10 to 100 amino acid residues, these peptides are net positive charge and membrane active. AMPs provide excellent antimicrobial or antibacterial effects, many AMP peptides also have antiviral and antineoplastic activities. Antimicrobial peptides play important roles in not only innate immune defense process, but also chemokine induction, chemotaxis, inflammation, and wound healing.
Introduction
Antimicrobial peptides are a heterogeneous group with 10-100 amino acids. There are more than 2600 AMPs in identification and registration database. All these peptides are produced by virtual organism like: bacteria, fungi, plants, and animals. Many vertebrate AMPs are secreted by epithelial surface cells, while some are expressed in neutrophils, monocytes, and macrophages.
Different types of antimicrobial peptides have the following commonalities:
- The number of amino acid residues is between 10 and 60 (average: 33.26)
- Almost all AMPs are cationic(average net charge: 3.32)
- Several anionic AMPs with aspartic acid and glutamic acid
Natural Distribution of AMPs
Antimicrobial peptides are in host defense mechanism against pathogen invasion, and preserved in eukaryotes. There are three plants-derived AMPs, including thionins, defensins and cyclotides. Plants-derived AMPs are rich in cysteine residues to form multiple disulfide bonds.
Thionins have cytotoxic effects on Gram-positive and Gram-negative bacteria, yeasts and fungi.
Most mammals have two AMP classes: cathelicidins, defensins.
Cathelicidins have high conserved cathelin domain with distinct peptide lengths, amino acid sequences and protein structures. Cathelicidins LL-37 is the only cathelicidin in humans.
Defensins are small cysteine-rich cationic peptides with three or four disulfide bridges. Depends on the spatial distribution of cysteine residues, three classification are distinguished in mammals: α-, β- and θ-defensins. Insert and mammalian defensins are active against bacteria, while plant defensins provides antifungal activity. The human β-defensin 3 (HBD3) also exists in the heart and skeletal muscle.
Action Mechanism of AMPs
Membrane Targeting Mechanism
The membrane-targeting mechanisms of AMPs including the pole and carpet models, and the pole model have two typical barrel-stave model and toroidal pore model.
Barrel-stave Model
Antimicrobial peptides aggregate together and penetrate the bilayer of membrane in multimers form. Then form channels and result in the cytoplasmic outflow. AMPs create a barrel-like pore within the bacterial membrane, the individual AMPs or AMP complexes is the staves form. The hydrophobic regions of AMPs point to the acyl chains of the membrane, while the hydrophilic areas form the pore.
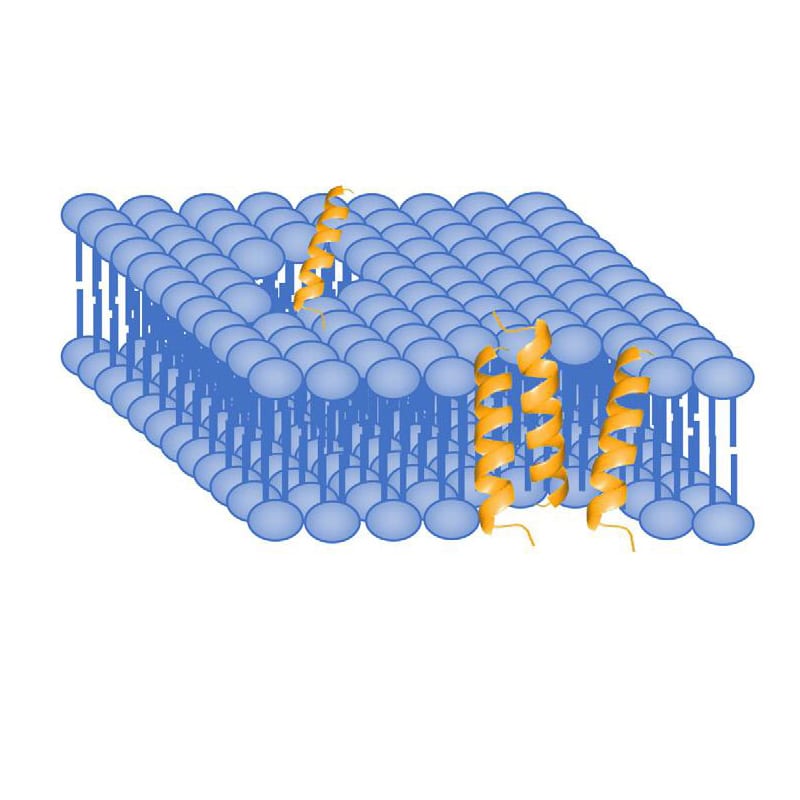
Toroidal Pore/Wormhole Model
Toroidal pore model, is also called wormhole model. AMPs embed vertically in the cell membrane, then bend to form the ring hole of 1-2 nm diameter. Different from Barrel-Stave model, the outer and inner leaflet of membrane are not in the trans-membrane channel.
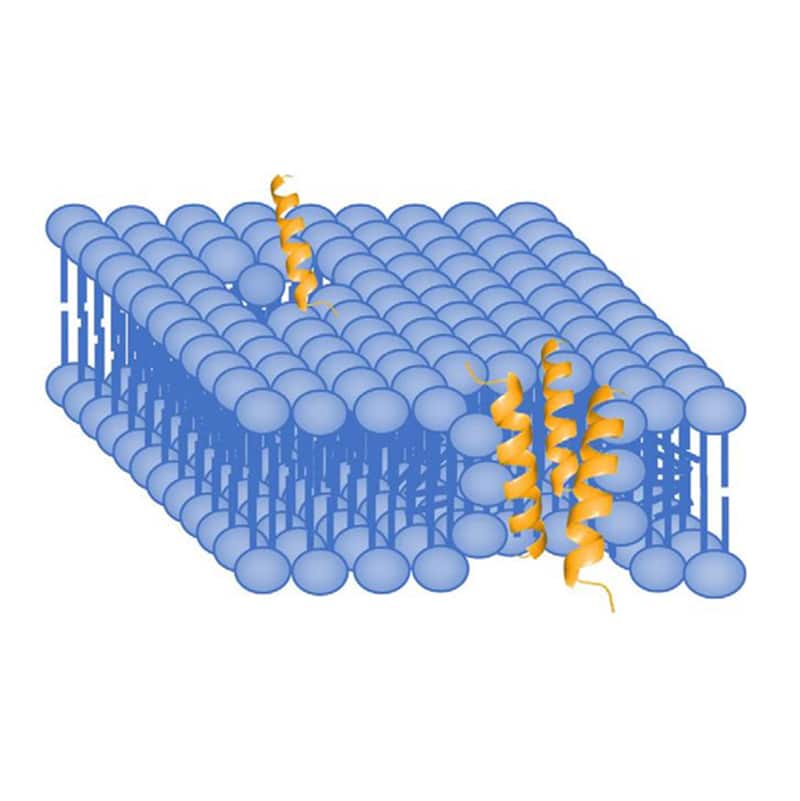
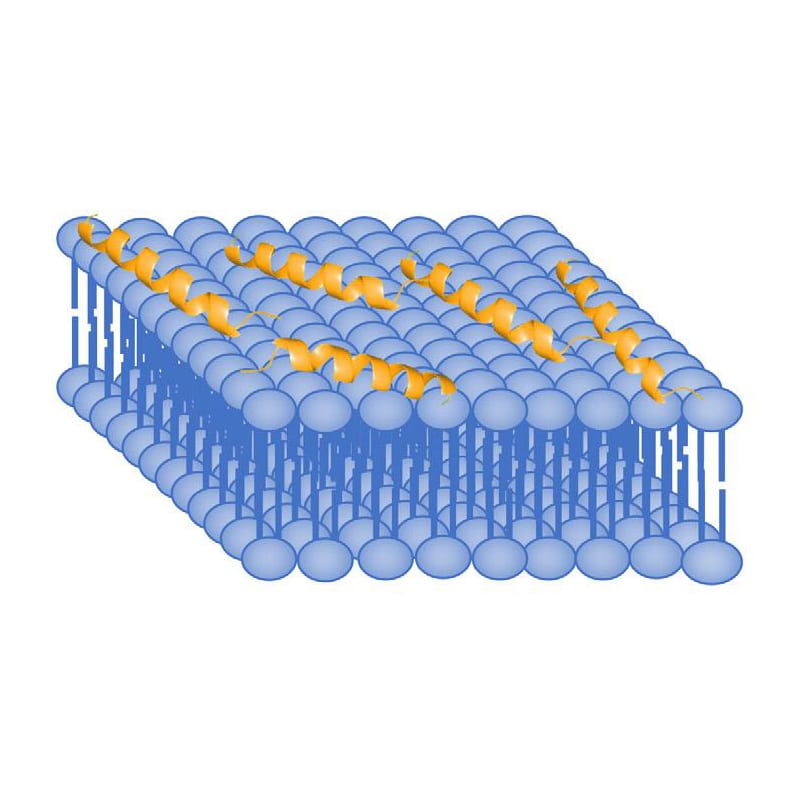
Carpet Model
AMPs arrange parallel to the cell membrane, the hydrophilic ends contact the solution, and hydrophobic ends contact the phospholipid bilayer. Then destroy the cell membrane in the detergent-like manner. In the Carpet model, AMPs cover the outer membrane surface at first, then disrupt the membrane by forming micelle-like units. Certain AMPs penetrate the membrane without channel formation, but inhibit nucleic acid or protein synthesis.
Antimicrobial peptides are able to be anti-biofilm agents, but these peptides are different from the cell penetrating peptides (CPPs). CPPs comprise 5 to 30 amino acids, typically, and can translocate acroos to the cell membrane. According to the physico-chemical propeties, the cell penetrating peptides have three categories: cationic, amphipathic, hydrophobic.
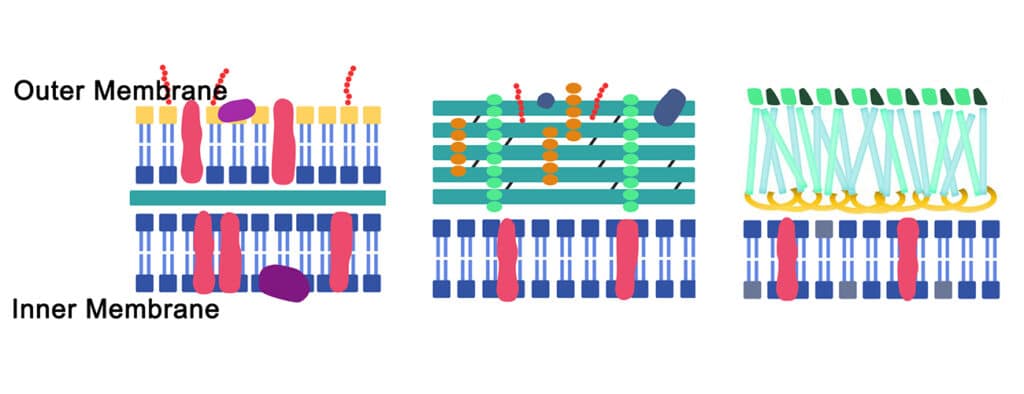
Anti-biofilm peptides have three different biofilm targeting mechanisms:
- Degradation of signals within biofilms
- Permeabilization within cytoplasmic membrane/EPS
- Modulation of EPS production
Non-membrane Targeting Mechanism
Direct penetration or endocytosis is the methods of AMP entering cells. Antimicrobial peptides identify and act on targets once it enters the cytoplasm. According to different targets, we divide AMPs into the following categories:
Protein Biosynthesis Inhibition
AMPs can affect transcription, translation, assembly of functional peptides by interfering molecular chaperone folding. Chaperones are key proteins for correct folding and assembling in protein synthesis with stereoisomerism. This make AMPs have cell selectivity and cytotoxicity prevention.
Inhibition of Nucleic Acid Biosynthesis
AMPs can affect key enzymes or induce the degradation of nucleic acid molecules, in order to inhibit nucleic acid biosynthesis. Indolicidin targets specifically the abasic site of DNA, it also can inhibit DNA topoisomerase. TFP1-1TC24 can degrade DNA and RNA after cell membrane rupture.
Protease Activity Inhibition
Many AMPs can inhibit metabolic activities by inhibition of protease activity. Cathelicidin BF can prevent aggregation of thrombin-induced platelet, and block protease-activated receptor 4.
Inhibition of Cell Division
Antimicrobial peptides inhibit cell division by prevention of DND replication and damage response, then block cell cycle and stop chromosome separation.
Classification of AMPs
The diversity of natural AMPs result in difficult classification, antimicrobial peptides are classified based on source, activity, structural characteristics, amino acid-rich species.
AMPs Sources Classification
The sources of antimicrobial peptides are divided into mammals, amphibians, microorganisms, inserts.
Mammalian Antimicrobial Peptides
Mammalian antimicrobial peptides exist widely in human, sheep, cattle, and other vertebrates. Cathelicidinss and defensins are the two main families of AMPs. As different position of disulfide bonds, defensins are divided into α-, β-, and θ-defensins.
Human host defense peptides (HDPs) provide different expressions in different stages of human growth. For example: Cathelicidin LL-37 (stock number) is usually detected in the skin cells of newborn infants. While Human beta-defensin 2(hBD-2, stock number) is often expressed in elderly. In addition to antibacterial activity, HDPs also affect immune regulations, apoptosis, and wound healing.
Amphibian-Derived Antimicrobial Peptides
Frogs are the main resource of amphibian AMPs, the most famous AMP is magainin.
Insect-Derived Antimicrobial Peptides
Antimicrobial peptides are normally synthesized in fat bodies and blood cells of insects. Cecropin is the most famous family of AMPs.
Microorganisms-Derived Antimicrobial Peptides
AMPs can be obtained from microorganisms like bacteria and fungi, the famous peptides are nisin, gramicidin.
Activity Classification of AMPs
Depending on the activity of AMPs, these categories are antibacterial, antiviral, antifungal, antiparasitic, anti-HIV (human immunodeficiency virus), antitumor (cancer).
Antibacterial Peptides (ABPs)
Antibacterial peptides have the broad inhibitory effect on common pathogenic bacteria. Many natural and synthetic AMPs (Nisin, Cecropins, Defensins) provide excellent inhibition activity to Gram-positive and Gram-negative bacteria.
Antifungal Peptides (AFPs)
Antifungal peptides can treat fungal infections with enhanced drug resistance. Many AFPs provide excellent antifungal activities against common pathogenic fungi.
Antiviral Peptides (AVPs)
Antiviral peptides have strong killing effect on viruses by the following mechanism:
- Inhibit virus attachment and virus cell membrane fusion
- Destroy the virus envelope
- Inhibit virus replication
Antiparasitic Peptides
Antiparasitic peptides provide the eradication effect on parasites with diseases induction. Such as malaria, leishmaniasis.
Anticancer Peptides (ACPs)
Anticancer peptides have different anticancer mechanisms as following:
- Recruit immune cells to perish tumor cells
- Induce the necrosis or apoptosis of cancer cells
- Inhibit angiogenesis to eliminate tumor nutrition and prevent metastasis
- Activate certain regulatory functional proteins to interfere the gene transcription and translation of tumor cells
Furthermore, both net charge and hydrophobicity also optimize the anticancer activity of ACPS.
Besides the peptide classification above, there are some peptides with bio-activity, but not same as antimicrobial peptides (AMPs):
- Besides the peptide classification above, there are some peptides with bio-activity, but not same as antimicrobial peptides (AMPs):
- Anti-inflammatory peptides: decrease the release of inflammatory mediators and cytokines, inhibit inflammatory signals.
- Anti-diabetic peptides: modulate the G protein-coupled receptor kinase, activate glucagon-like peptide-1 (GLP-1), glucagon receptors.
Amino Acid-Rich Species Classification
Proline-Rich Peptides (PrAMPs)
Proline is a typical non-polar amino acid, PrAMPs normally enter bacterial cytoplasm by the inner membrane transporters SbmA. PrAMPs target ribosomes once in the cytoplasm, then block the binding of aminoacyl-tRNA to peptidyltransferase center, or trap decoding release factors in termination of translation. In order to interfere the protein synthesis.
Tryptophan/Arginine-Rich Peptides
Tryptophan (Trp) is a non-polar amino acid, it has remarkable effects on the interface region of the lipid bi-layer. While Arginine (Arg), as a basic amino acid, confers peptide charge and hydrogen bond interactions. Indolicidin and Triptrpticin are two famous AMPS with Arg and Trp -rich residues.
Histidine-Rich Peptides
Histidine is a common basic amino acid, the histidine-rich AMPs have good membrane permeation activity. HV2 is a histidine-rich AMP, it increases the permeability of bacterial cell membranes, and result in cell membrane rupture and death.
Glycine-Rich Peptides
The R group of glycine is a non-polar amino acid in biology. Glycine residues have the critical effect on the tertiary structure of peptide chains. The glycine-rich AMP activates the phagocyte-mediated microbicidal mechanisms.
Conclusion
Different structure of AMPs represent the unique source for targeted exploration in the therapy of microbial and viral infection, cancer and sepsis. Qyaobio can apply modern synthetic methods to produce accurate compounds and peptides candidates. The achievements in peptide library, MS/HPLC analytical method, screening and formulation technologies, will contribute to solve intrinsic issues in AMPs therapeutic.
QYAOBIO has considerable expertise and long-standing experience in peptide synthesis. Our excellent capacity of custom and modified peptide synthesis, make us to be the reliable partner in worldwide.