Antigenic Peptides Design
Introduction
Antigenic peptides are synthesized via short amino acid sequences derived from carefully selected native target proteins. They can be used as immunogens to produce custom antibodies and are a popular option for using full-length proteins as immunogens. Their application has grown significantly in recent years.
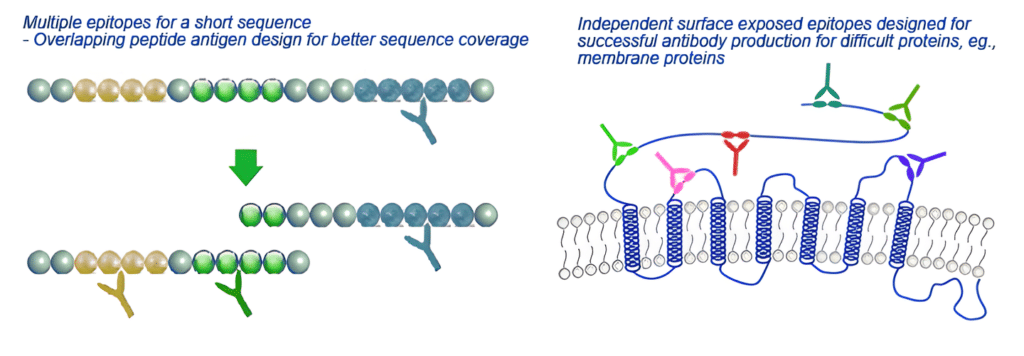
Advantages of Using Peptide Antigens
First, peptide antigen is a excellent choice to generate antibodies when the amino acid sequence of the target protein is known, but the protein itself is unavailable, or a structurally stable and complete protein cannot be obtained due to the structure is too complex such as certain transmembrane receptors, ion channels. Second, peptide immunogens have the added advantage of providing flexibility for selection and antigen design of target protein domain.
Additionally, since its ability to limit the number of potential epitopes, peptide antigen is an ideal antigen to detect post-translational modification (PTM) sites within target proteins. Although the drawback of most antibody projects and applications is often fewer potential epitopes, the limited number of epitopes facilitates the identification of reactive antibodies that specifically target the PTM site when making PTM antibodies.
Peptide antigens also provide the following benefits:
- The epitope is precisely defined from the beginning.
- It is sufficient to provide only the protein sequence.
- less possibility of cross-reactivity.
- Antigens are completely synthetic, inexpensive, and often available for a short time
Peptide Antigen Options
Multi Antigenic Peptides (MAPs)
Multiple antigenic peptides (MAPs) are peptides derived from artificial branches. Lysine residues serve as the scaffold core and are covalently linked to up to eight branches with identical or different peptide sequences. For a very long period, MAPs have been utilized in immunological research to produce antibodies. New architectures such concentrated branching peptides can significantly boost the immune response, even though certain peptides have a lesser immunological response. Standard SPPS can be used to synthesis a large number of antigenic peptides. This procedure involves anchoring Boc-Lys(Boc)-OH to a resin, treating the peptides with TFA, and carrying out sequentially successive cycles of coupling and deprotection. After this, each of the eight branches is produced to contain peptides that will be employed in immunological research.
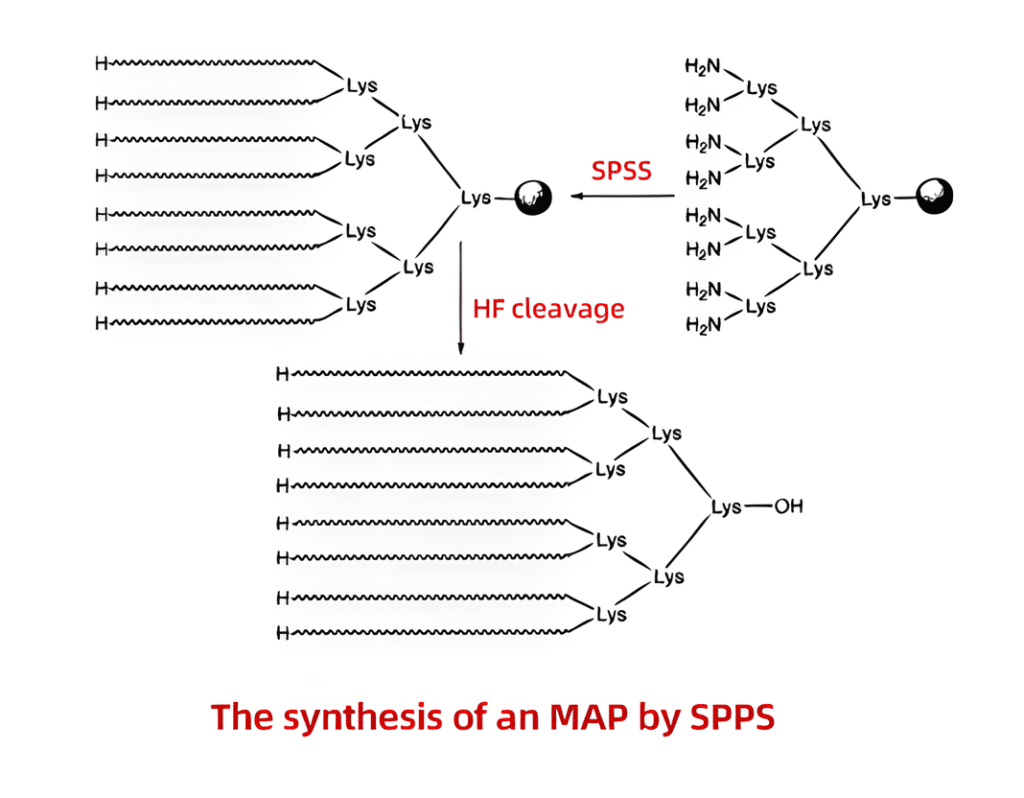
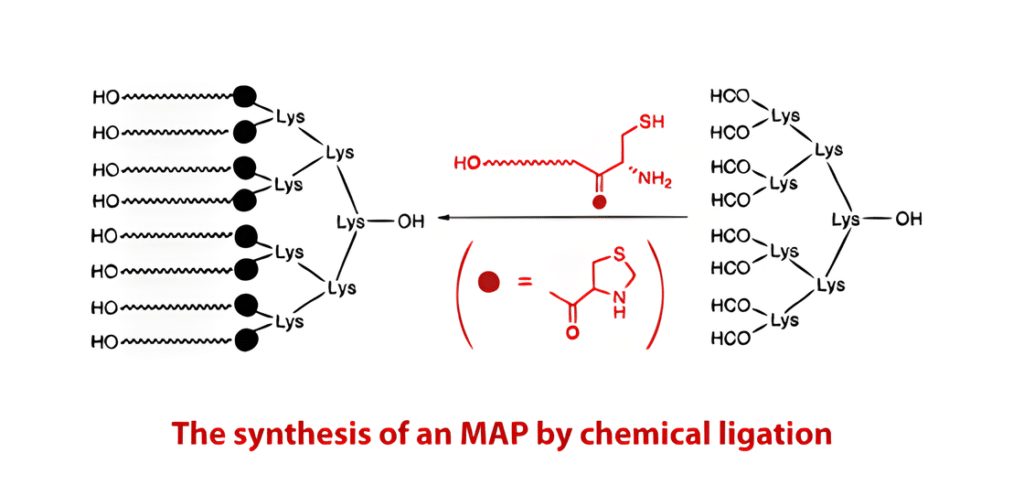
However, MAP synthesis can be difficult because it is uncertain what branching proteins are made of. For instance, the peptides may aggregate on the resin due to the gap between the eight branches, which could lead to low coupling yields and/or deletions. This problem can be solved by chemical ligation using PeptideSyn technology. This makes it possible to generate the necessary peptide dendrimer at yields that are higher than what would be possible with conventional techniques. The protein’s increased molecular weight as a result of the branching structures promotes immunogenicity.
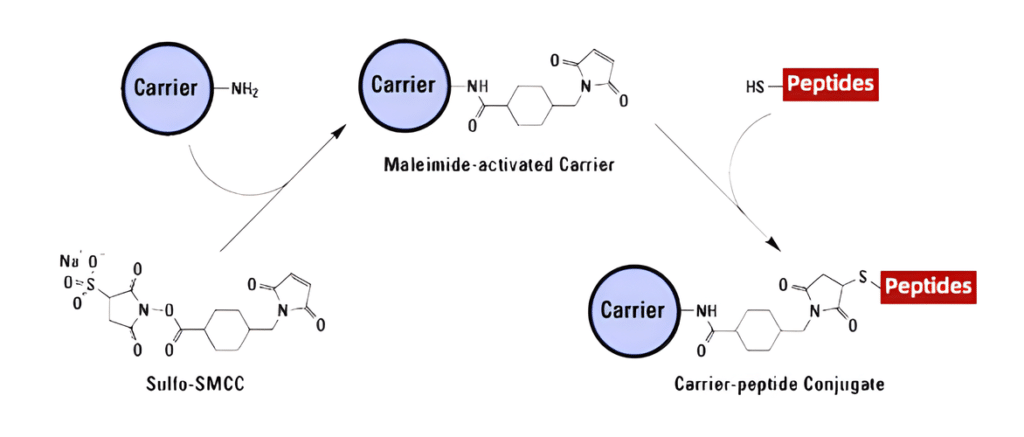
Peptide-carrier Protein Conjugation
Since most peptides are too small to elicit an immune response to result in the production of antibodies, the peptide of interest is combined with carrier proteins that contain many epitopes to stimulate T-helper cells. In this process, peptides are synthesized in a conventional manne with a Cys group at the C- or N-terminus, subsequently conjugated to the carrier protein in a separate process. Several common carrier proteins used in antibody production are as follows.
KLH (keyhole limpet hemocyanin)
It is a copper-containing protein that was identified from Megathura crenulata and is found in mollusks and arthropods. KLH has great immunogenicity in rabbits, making it the most often utilized carrier. Peptide-KLH is commonly used for immunization, whereas peptide-BSA is used for ELISA tests.
BSA (bovine serum albumin)
One of the most stable and soluble albumins in cattle is this plasma protein. There are 59 lysines in BSA. For linker conjugation, about 30–35 of these primary amines can be employed. Antisera against peptide-BSA have the potential to produce false positives when the BSA is used as a blocking buffer reagent.
OVA (ovalbumin)
It is a protein that has been separated from egg white. If antibody is specific for that peptide, it acts well as a second carrier protein.
Design Strategies for Peptide Antigens
While numerous peptide sequences have the potential to elicit an immune response, not all of them are equally successful in producing antibodies that are reactive to the target protein. Peptide antigen-based antibody production success is dependent on a number of variables that must be taken into account throughout the design process, including:
- The precision of the amino acid sequence of the target protein
- The whole protein’s expected 2ary/3airy structure
- Protein domains/regions mimicked by peptide antigens are selected
- Peptide antigens need to be conjugated to larger carrier proteins
- The simplicity of synthesizing certain peptide sequences
Consequently, the following general suggestions relate to the design of peptide antigens:
- Verify that the protein sequence and species have been recognized correctly.
- Select the peptide antigen from a natural protein region that is accessible to solvents. These are frequently the areas of the protein surface that are exposed and come into contact with the solvent’s aqueous (hydrophilic) environment. Antibodies may not be able to access specific parts of a protein, including transmembrane regions or the center of globular proteins.
- The ability to design peptide antigens to target a specific epitope of interest is a significant benefit. According to the specific protein database, the candidate of peptide antigen was screened. There’s a chance that some domains are also found in other proteins. You should steer clear of these sequences to reduce the possibility of unwanted cross-reactivity and maximize your overall antibody specificity. In order to decrease undesired off-target protein binding, the ideal sequences to choose are those with minimal sequence homology.
- Based on the three-dimensional structure of the target domain in the intact protein, constraining techniques may be required to design and synthesize conformationally constrained peptide antigens in order as the tertiary structure of the antigen was optimally mimiced.
- Without first conjugating to a carrier protein like KLH, BSA, or OVA, peptide antigens are frequently too small to trigger an adequate immune response. Peptide-carrier protein conjugates are usually advised as a result.
Synthesis of Peptide Antigens
The simplicity of synthesizing specific peptide sequences should be taken into account while designing peptide antigens. It is suggested that both hydrophobic or hydrophilic residues should be included, and amino acids that facilitate immunogenicity, such as basic and aromatic amino acids, are incorporated into the peptide. It is preferable to avoid long chains of hydrophobic residues and to keep the content of hydrophobic amino acids below 50%. Every five amino acids should contain at least one charged residue (lysine, glutamine, aspartic acid, or arginine) for peptide solubility. Conservative substitutions or the insertion of polar residues to the N- or C-terminus can also increase the solubility of peptides. When designing antigens, it is preferable to steer clear of complicated regions like beta-sheets and alpha-helices ,but instead target flexible regions.
Conclusion
Peptide antigens are the best option when it comes to producing monoclonal and polyclonal antibodies. For the synthesis of peptide antigens, Qyaobio uses its cutting-edge skills and capabilities, which include conjugation, PTMs, conformational constraints, or biotinylation if you required. Peptide antigen should have a purity of at least 85% for the majority of antibody production projects. On the other hand, we can also supply antigens with higher purities upon request.







