Antimicrobial Peptides
Antimicrobial peptides (AMPs) are a diverse classification of small peptides.
Antimicrobial peptides (AMPs) are a diverse classification of small peptides in nature organism, these peptides have a critical role in the immune system, no matter in innate or acquired responses to pathogenic attacks. Antimicrobial peptide are released by different cells and organelles, including immune cells (granulocytes and macrophages), epithelial cells (aginal epithelium, respiratory epithelium, and oral cavity epithelium), and small intestine cells.
Both synthetic and natural antimicrobial peptides demonstrate the broad-spectrum antimicrobial activity with high specificity and low toxicity. These peptides have distinctive structures and functions for sophisticated action mechanisms.
Antimicrobial peptides are short peptide with 10 to 50 amino acids, this classification peptides have a broad spectrum of antibiotic activities against bacteria, yeasts, fungi and viruses. In addition, AMPs also have cytotoxic activity on cancer cells, anti-inflammatory, and immunomodulatory activities. Antibacterial peptides are the critical tool for antimicrobial drug development, the remarkable effects of AMPs including:
Antimicrobial Peptide Structure
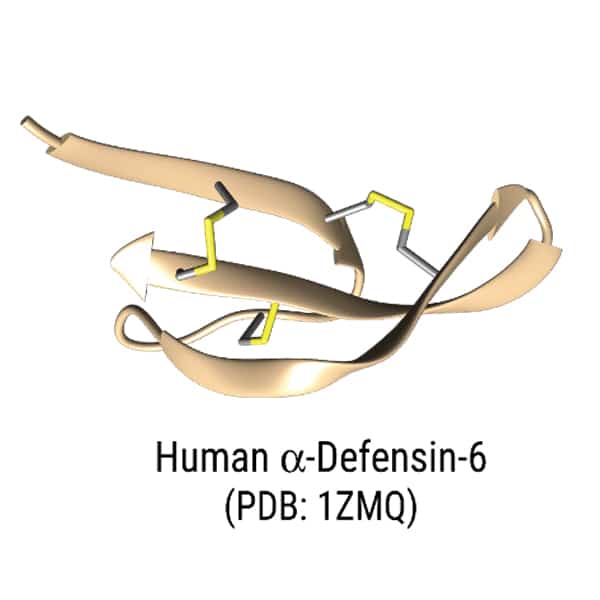
The unique sequence of antimicrobial peptides determine the distinct activity, these sequences consist of more than 50% hydrophobic residues. Normally, antimicrobial peptides are cationic in nature, and contain modification like cyclization or disulfide bonds.
Antimicrobial peptide have various structural motifs, the common secondary structures of antimicrobial peptides are alpha-helices, beta-sheets, cyclic loops, and extended structures. Different structural motifs and properties provide different modes of antimicrobial mechanism.
Antimicrobial Peptides Mechanism
As most AMPs are plycationic with charges from +2 to +9, this enables them to attract electrostatically to the negatively charged surface of bacterial membranes. In gram-negative bacteria situation, antibacterial peptide can bind the anionic phosphate groups in outer membrane. However, gram-positive bacteria have neither outer membrane nor lipopolysaccharides to bind AMPs. These bacteria have peptidoglycans on the surface to attract AMPs by anionic teichoic acid presentation. Furthermore, lipopolysaccharides (LPS) and peptidoglycans on bacteria surface enable antibacterial peptides to distinguish bacteria from host cells. This make AMPs have considerably less toxic than the traditional molecule antibiotics.
The AMP interaction mode with membranes depends on the charge distribution, shape, and secondary structures. The common action mode of AMPs to trigger apoptosis or necrosis involve:
The AMP interaction mode with membranes depends on the charge distribution, shape, and secondary structures. The common action mode of AMPs to trigger apoptosis or necrosis involve:
- Targeting of bacterial cellular membranes: membrane lysis or disruption
- Intercellular proteins and membranes: endoplasmic reticulum membrane
Targeting bacterial cellular membrane
The AMP insertion or disruption of bacterial cellular membranes start from electrostatic interactions. Following this electrostatic pairing interaction, the absorption of AMP occurs in the outer bacterial membrane. Hydrogen-bonds formation between basic amino acid residues in antibacterial peptides, and phosphate groups on membrane surface. This action will break down the salt bridges between phosphate groups and neighboring cations.
In addition, hydrophobic interactions between uncharged residues (leucine, isoleucine, valine, trytophan, phenylalanine) and hydrophobic carbon chains of Lipopolysaccharides (LPS), will further disrupt the close packing structure and cause more disorganization in the membrane. This process is called membrane thinning. Once the AMPs concentration reach to a critical level around the bacterium, thin membranes cause lateral expansions and increase water translocation. Finally, this disruption results in cell death by membrane leakage and reduced potentials.
Pore formation
Pore formation can result in rapid cell death by expelling of intracellular components. The structure of AMP will affect the formation of pores. There are two main models: barrel-strave, toroidal.
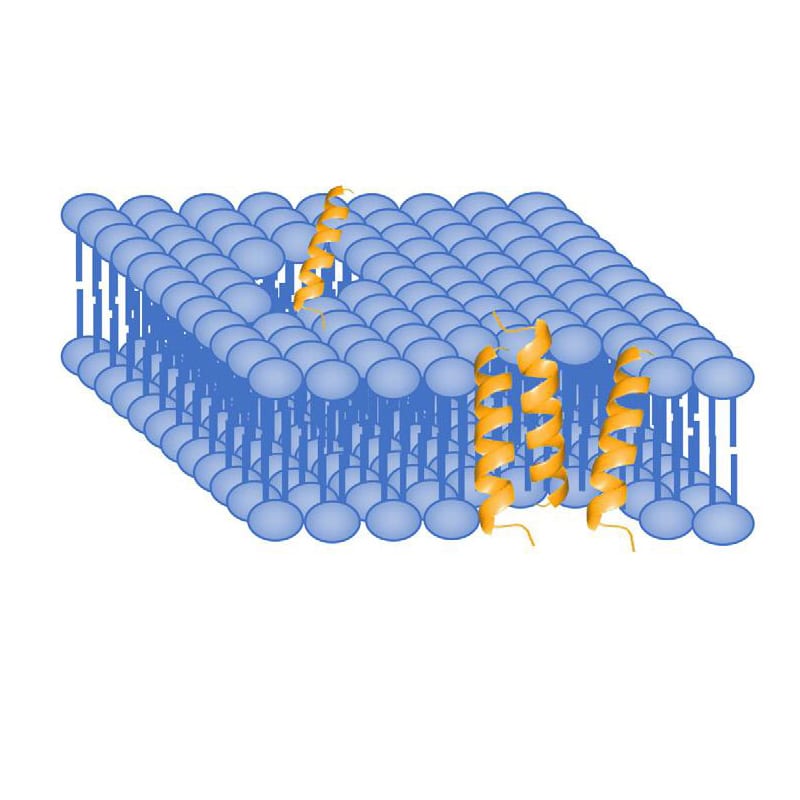
In barrel-stave model, AMPs aggregate and insert into the membrane bi-layers, then hydrophobic regions of peptides associate and align with hydrocarbon core of lipids by process of hydrophobic matching. Therefore, the hydrophilic regions of AMPs form the pore on the interior surface, and allow appropriate hydrophilic molecules to move through freely.
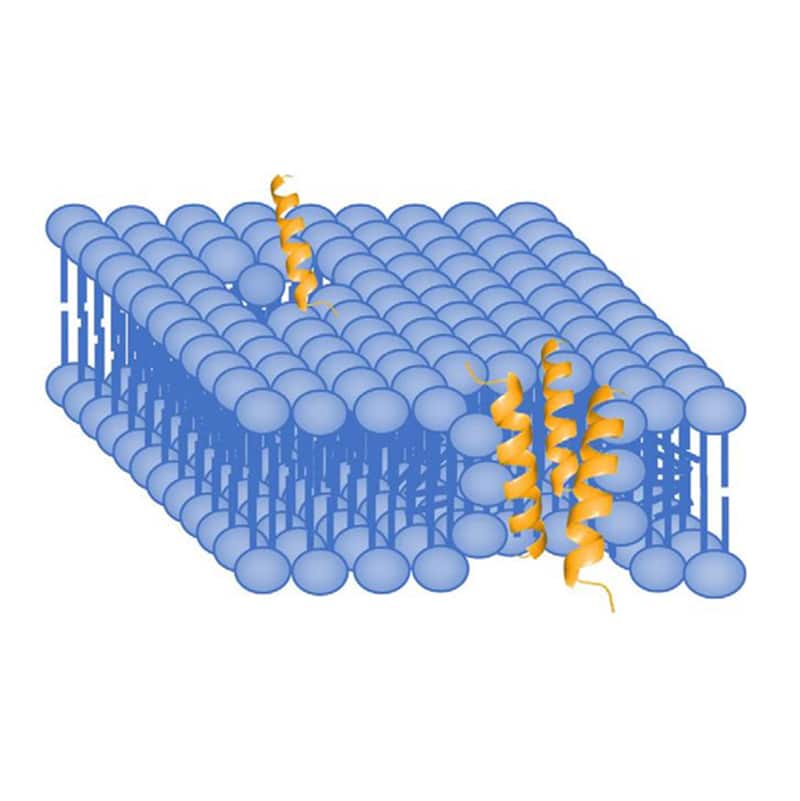
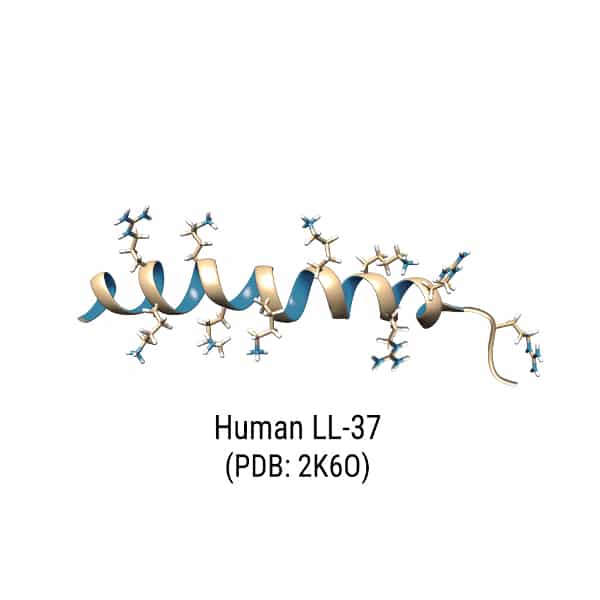
In the toroidal pore model, AMPs penetrate deeper into the membrane. Because of the elecrostatic interactions with the polar head groups in lipid bilayer, antimicrobial peptide will cause membrane curvature and packing disruption. LL-37 is a good example of AMP with induction of 2 to 3 nm diameter-sized pore. Normally, the pore size is depended on AMP concentration, low concentrations only form transient pores, while higher concentrations form more stable pores.
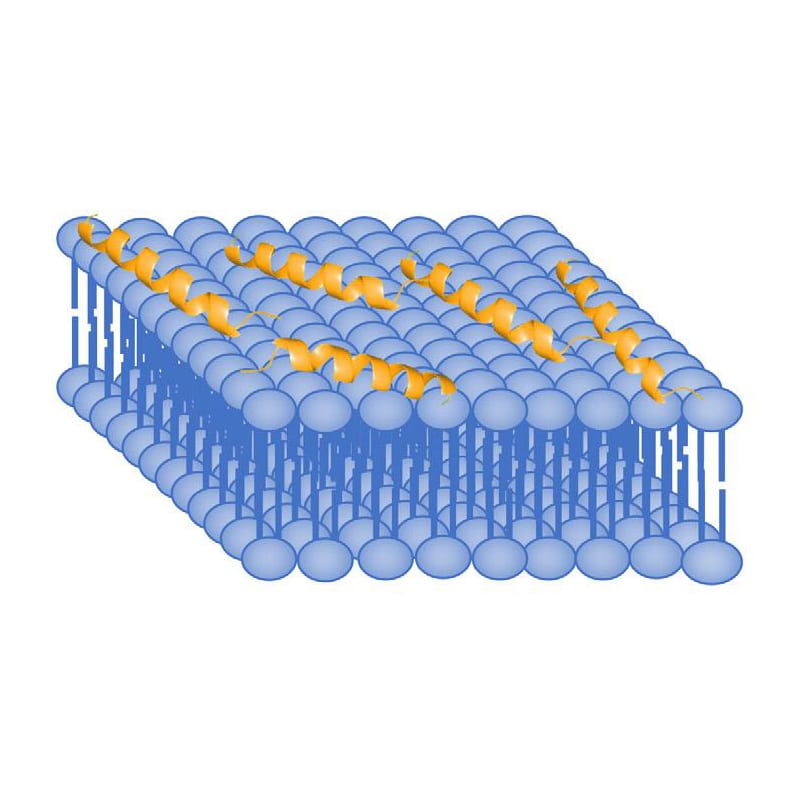
The formation of membrane pores depends on AMP ability, there are two main permeabilization mechanism: carpet model, pore model. The carpet model requires high AMPs concentration to cover bacterial cells for high efficiency. While the pore model only require lower concentration, this makes pore mechanism more attractive for therapeutic targets.
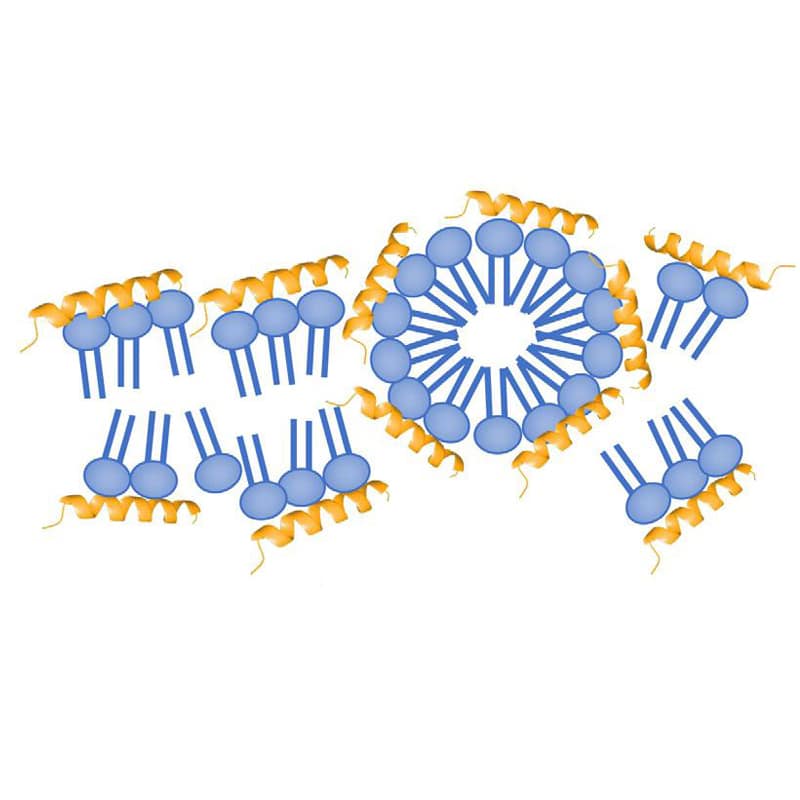
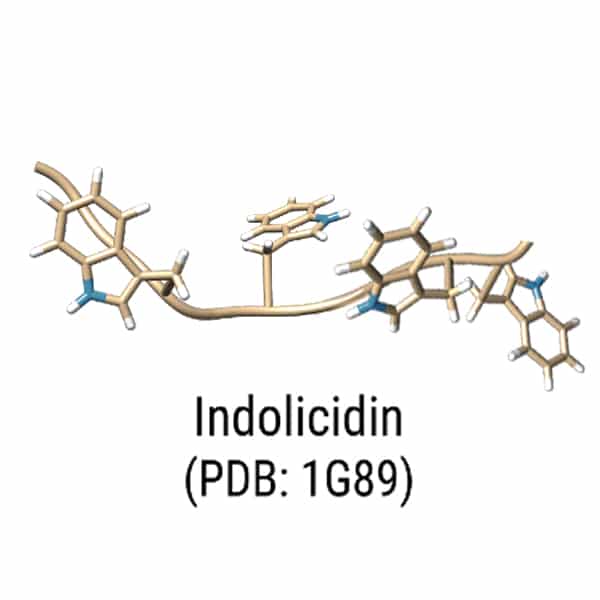
The killing modes of AMPs are not only membrane disrupting and puncturing, some AMPs (indolicidin, HNP-1, dermaseptin) can inhibit certain critical cellular processes without membranes disruption. Although many AMPs have dual mechanism with both membrane disruption and apoptosis, some AMPs only appear in activity once entering the cell. Such as Coprisin, an analog of defension with broad-spectrum antifungal activity without any human erythrocytes harm.
Cell Selectivity of AMPs
The cationic AMPs interact selectively with negative charge membrane by electrostatic interaction. Furthermore, cancer cells exhibit more negative charge PS outside membrane. In addition, ther are a large number of microvilli on membrane surface of cancer cells, this increase the binding area for AMPs. The cell surface architectures between mammalian and bacterial cell are different in composition and electrostatic charge. Therefore, drugs can recognize this difference have the potential to be antimicrobial therapeutic. Antimicrobial peptides kill invading microbes selectively without significant human cell harming.
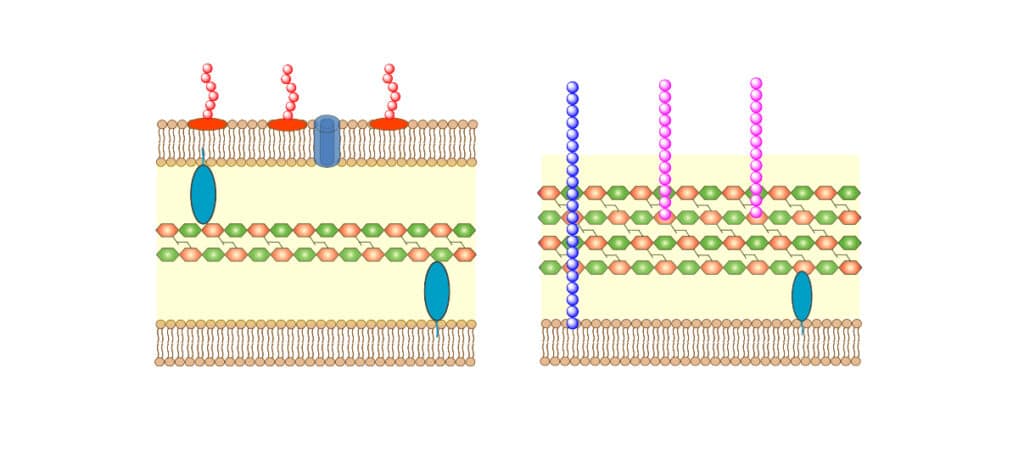
Comparing to bacteria cellular membranes, mammalian cytoplasmic membranes are rich in phospholipids, like phosphatidylcholine (PC), phosphatidylethanolamine (PE), and sphingomyelin (SM). This zwitterionic in physiological conditions make the membrane neutral in charge.
Antimicrobial peptide with cancer cell target disrupts the membrane by pore-forming and membrane-thinning mechanism, this is similar to AMP-bacteria interactions. LL-37 has well-studied value in anticancer therapeutic, the interaction between cancer cells and LL-37 are complicated. Depending on different cancer type and tissue affection, LL-37 may have contradictory effects.
Call Us
+86(021)-50795728
+86(027)-60707970