CDR-H3/C2
The hypervariable loop structures of the antigen-binding parts of antibodies are called Complementarity-determining regions (CDRs), which decide the specificity of antigen binding. Site-directed mutagenesis of individual amino acid residues in the antibody CDRs can be used to improve antibody affinity. CDR-H3/C2 is a 16 amino acid peptide, which derived from the third heavy chain domain and modified by cyclization. It has the ability to prevent HIV-1 replication.
Initially, the sequences of mouse monoclonal antibody F58 that can neutralize HIV-11 were employed to construct CDR-H3/C2. It is a member of the immunoglobulin superfamily of peptides, and its amino acid sequence is CDLIYYDYEEDYYFDC. A disulphide bond holds the ends of the sequence of CDR-H3/C2 together to form a cyclic structure. Effectively binding to HIV-1 variable region V3, CDR-H3/C2 is likely to inhibit the interaction of HIV-1 engage with host cell antibodies. Conversely, this prevents HIV from infecting the host cell. Therefore, it is considered a neutralizing peptide for HIV-1.
Notice: All peptides are only for research purposes, Not for clinical use.
CDR-H3/C2
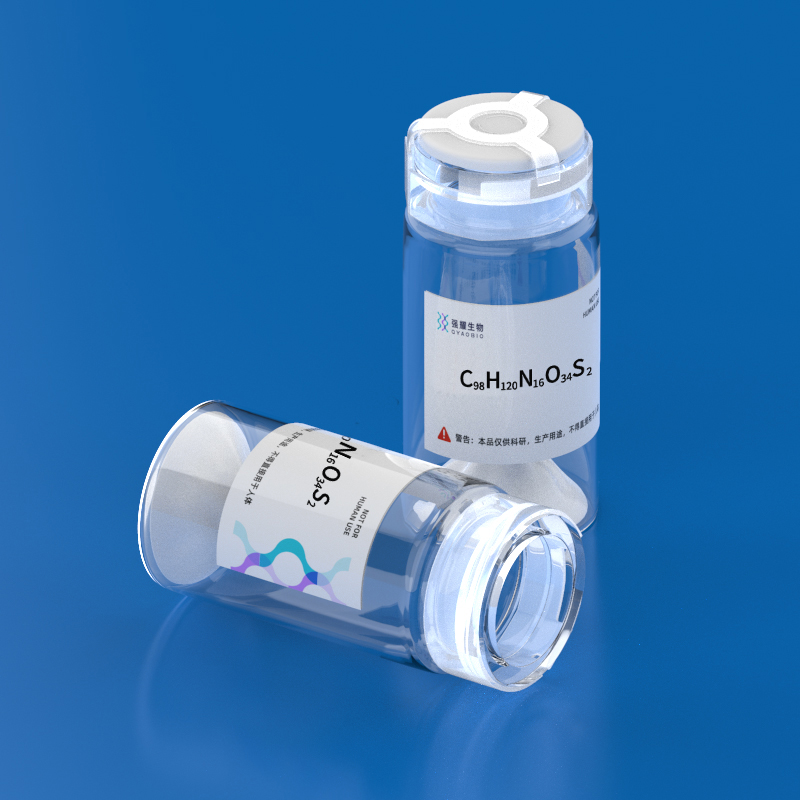
- Name: CDR-H3/C2
- Sequence: CDLIYYDYEEDYYFDC
- CAS Number: None
- Formula: C98H120N16O34S2
- Characteristics: (Cys1-Cys16 bridge)
- Reference: None
Call Us
+86(021)-50795728
+86(027)-60707970
