Chemo Enzymatic Peptide Synthesis
Introduction
Chemo enzymatic peptide synthesis (CEPS) is a cutting-edge technology with unique versatile engineered enzymes. It is difficult to synthesize long and complex peptides by traditional SPPS (Solid Phase Peptide Synthesis). Moreover, the chain length increases will result in high impurity levels. Furthermore, peptide recombinant expression, as the alternative method, cannot guarantee the finally soluble products.
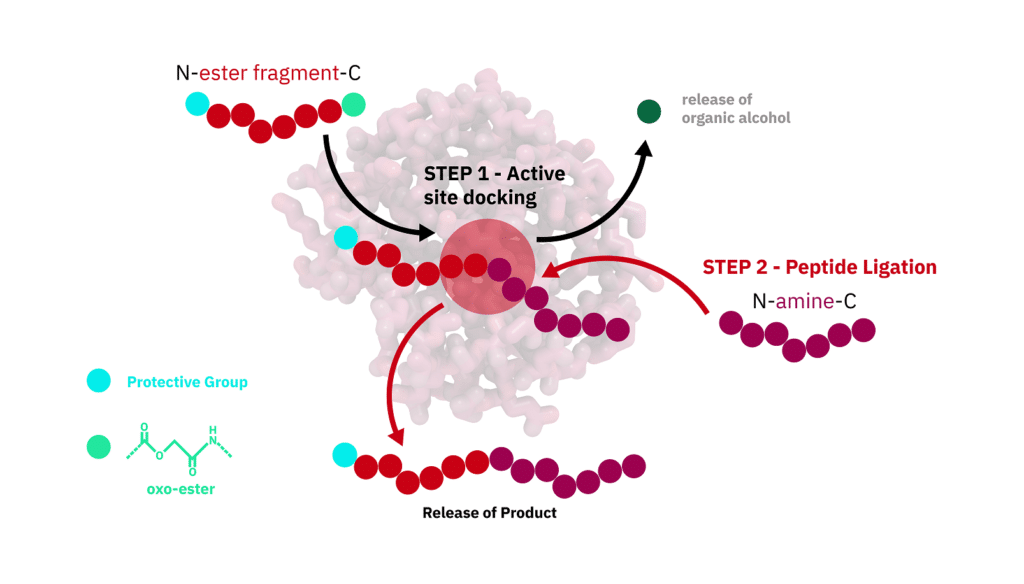
While CEPS can overcome all these issues and ligate peptides together without traces. It is the simplest method to generate long peptides with active conformation without recombinant production. CEPS is ideal for peptide production up to 160-200 amino acids in length.
CEPS is the new synthetic route for peptide-based new chemical entities (NCEs), small proteins, peptide-containing bio-conjugates. Chemo enzymatic peptide synthesis is the hydrolase-catalyzed stereoselective formation of peptide bonds. Comparing to conventional chemical synthesis with harsh reaction conditions, it is a clean and mild procedure without complicated and laborious protection-deprotection procedures.
Recently, the recombinant techniques, substrate mimetics, and optimal reaction conditions can produce a variety of enzymes and substrates. This broad the scope of chemo enzymatic peptide synthesis.
CEPS VS SPPS
Both linear and circular peptides are important target molecules for pharmaceutical, nutritional, and cosmetic applications. Although there are different peptide synthesis methods, like solid phase peptide synthesis (SPPS) and recombinant bacterial expression systems. These methods have severe drawbacks.
- SPPS technology can control the amino acid sequence precisely, but the laborious protection-deprotection regimen, harsh reaction conditions and toxic reagents make this procedure difficult.
- In the recombinant expression system, the yield rates are very low, and the purification procedures are time-consuming.
CEPS provides a flexible and more specific method for peptide synthesis. It has enzymatic reactions in mild conditions. More importantly, enzymatic chemoselectivity can achieve the conversion of non-side chain protected peptides and the absolute absence of recemization.
Key Features
- CEPS generate longer peptides over the range of SPPS in 80 AAs
- Significantly two times higher yields than SPPS
- Pres-purified fragments for ligation lead to higher purity product than SPPS
- Reduce associated cost in peptide synthesis significantly
- Wide applications in the discovery filed by linking all sorts of payloads via short peptides
- Backbone cyclized peptides with large ring size of more than 12 AAs
- Possible cyclotides with 3 disulfide bridges
CEPS Technology Benefits
| CEPS | Recombinant |
| Long peptides and synthetic proteins with unnatural amino acids or other functionalities | Impossible of unnatural amino acids or other functionalities |
| Rapid production of proof-of-concept molecules, nominative testing of ideas and candidates | Time consuming and complex production |
| New variants with high success rate in rapid production | Lengthy cloning and expression with solubility or folding issues |
| C-terminal amide peptide for increased stability | Impossible of C-terminal amide |
CEPS Reaction Mechanisms
The enzymes function in Chemo enzymatic peptide synthesis (CEPS) is optimal in their native environment. The unique catalytic abilities depend on the PH, substrate concentration, and organic solvents.
Generally, protease has hydrolysis efficiency, but it can function in opposite direction with peptide bonds (aminolysis) formation. The hydrolysis and aminolysis compete with each other, while in certain specific condition, enzymes prefer aminolysis to hydrolysis.
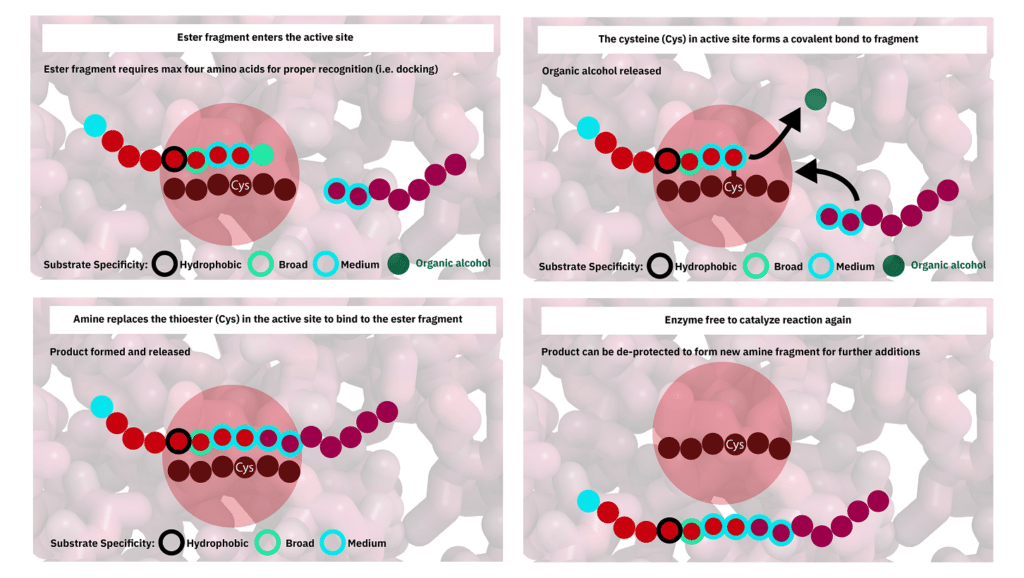
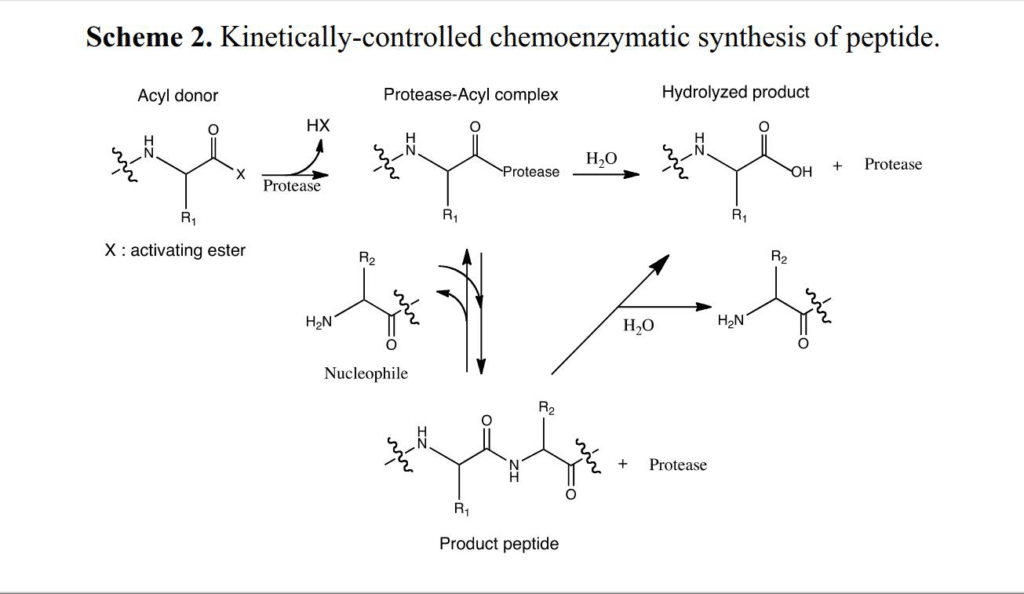
Protease-catalyzed peptide synthesis is either equilibrium-controlled or kinetically controlled. In equilibrium-controlled synthesis, all proreases are consumed, the free carboxyl groups act as acyl donors. The protesase only improve the reaction rate, but never affect the final equilibrium.
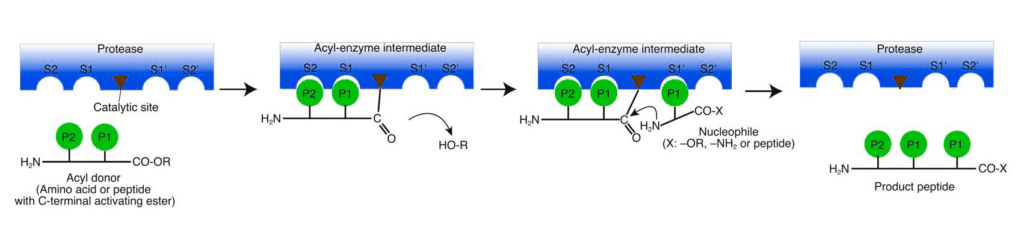
Comparing to the kinetically controlled reactions, The major drawbacks of the equilibrium-controlled synthesis are low yield and slow reaction rate. In kinetically controlled synthesis, the activated esters are required for the acyl group donation. The effect of hydrolysis increases as the reaction substrate decreases, it is critical to stop the aminolysis reaction before hydrolysis rate exceed aminolysis rate.
Enzymes in CEPS Peptide Synthesis
In chemoenzymatic peptide synthesis, there are different enzymes, such as cysteine protease, serine protease, esterase. In addition, engineered proteases are synthesized by random mutagenesis, site-directed mutagenesis, and encapsulation in silica nanospheres, in order to enhance the activity, stability, and selectivity.
Cysteine Protease
Papain
Papain is the proteclytically active constituent in the tropical papaya fruit, it exhibits the activities of endopeptidase, amidase, and esterase. In addition, papain has broad substrate specificity, it is stable and active in conditions from pH 4 to pH 10 at temperature up to 80 ℃. Papain-catalyzed synthesis applies the l-alanine ethyl ester as the monomeric substrate for poly l-alanine. The protease-catalyzed co-polymerization of amino acids are achieved by papin with substrates of l-glutamic acid ester and various amino acid esters.
Bromelain
Bromelain has a broad specificity for the protein cleavage, especially for polar amino acids in P1 position. The optimal pH range for protein substrates is broad. Comparing to other proteases, bromelain can give the highest yield and average chain length.
Serine Protease
α-Chymotrypsin
Chymotrypsin is the major protease component of pancreatic juice, the major cleavage sites are peptide bonds with the carboxyl side is a hydrophobic amino acid, for example tyrosine, tryptophan, and phenylalanine. All these amino acids contain the aromatic ring in side chains, which fits into a hydrophobic region of the enzyme.
Proteinase K
The designation of K indicates its hydrolyzation of native keratin. Proteinase K has wide broad substrate specificiy, it can cleave peptide bonds next to the carboxyl group of aliphatic and aromatic amino acids, and hydrolyzes esters. In addition, Proteinase K is active in wide pH ranges from 7.5 to 12.0, and at high temperatures to 60℃. The first proteinase K-catalyzed peptide synthesis is in linear oligo(l-phenylalanine) and star oligopeptides with branching terminator of tris(2-aminoethyl)amine.
Trypsin
Trypsin has proteclytic activity in pancreatic secretions, it has specificity towards the C-terminal peptide bond of lysine and arginine residues, except the -Arg-Pro- and -Lys-Pro- bonds. pH 8 is optimal for the activity of trypsin.
Subtilisin
Subtilisin (alcalase) is an extracellular serine endoprotease, it has excellent stability in the pH of 7-10. Subtilisin-catalyzed strategy is established in neat organic solvent with large peptide fragments up to 10-mer level.
Lipase
Lipase can catalyze the hydrolysis of triglycerides. In addition, it also can catalyze other reactions like esterification, amidation, and transesterification. Lipases can accept various substrates while maintain the regioselectivity and stereoselectivity, and are highly stable even under adverse conditions.
Engineered Proteases
Site-directed mutagenesis is used to design trypsin variants with decreased side reaction rate of proteolysis. Such as: the stability of α-chymotrypsin with methionine sulfoxide modification remains at 80% for 4 hours in pH 9. α-chymotrypsin methylated at the ε2-N also shows efficient catalysis in peptide synthesis.
Applications of CEPS
Metal-chelating Agent
Some peptides synthesized by chemoenzymatic technology have metal chelating abilities. Phytochelatins peptides consist of alternating γ-glutamic acid and cysteine residues, these residues can bind to heavy metals for cellular metal detoxification. Phytochelatin-like peptides are normally synthesized by employing papain-catalyzed oligomerization, the binding ability of synthesized peptides to heavy divalent metals are estimate 50% of the perfect sequence.
Antibiotics
CEPS peptides are applied as antibiotics in different research areas. Puromycin is a minoacyl nucleoside antibiotic, it is an inhibitor of protein synthesis. The aminoacyl nucleoside is classified as a mimic of aminoacyl-tRNA. Puromycin analogues are applied in the development of new antibiotics or chemical probes for biochemical studies. The engineered aminolysin-A can catalyze the synthesis of diverse puromycin analogues with distinct amino acid residues. Moreover, all these engineered analogues have similar antimicrobial activity as the natural puromycin.
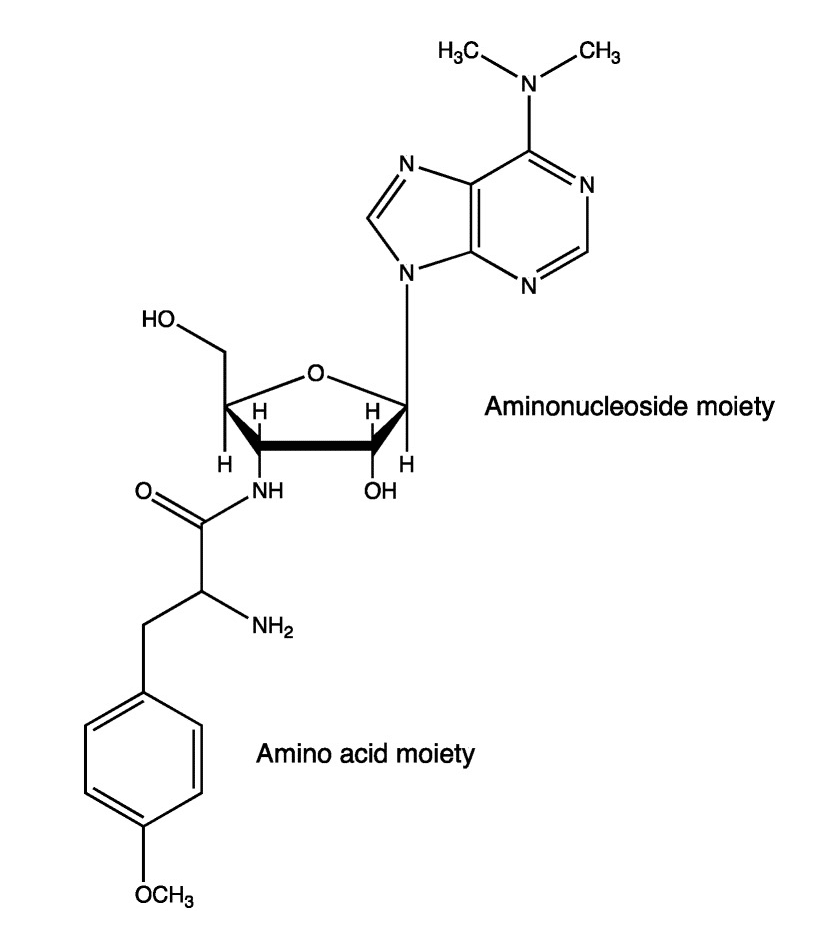
Cyclotides are the unique class of head to tail microproteins with cyclic cysteine-rich, these kinds of long peptides have length up to 37 amino acids. Cyclotides have a wide range of biological properties, from anti-HIV to insecticidal.
Surfactants
Gemini or dimeric surfactants have several unexpected properties superior to the conventional monomeric surfactants, such as excellent solubilizing, wetting, foaming, and antimicrobial properties. In addition, these novel surfactants have great potential for synergism, in order to improve efficacy and effectiveness of other surface-active compounds.
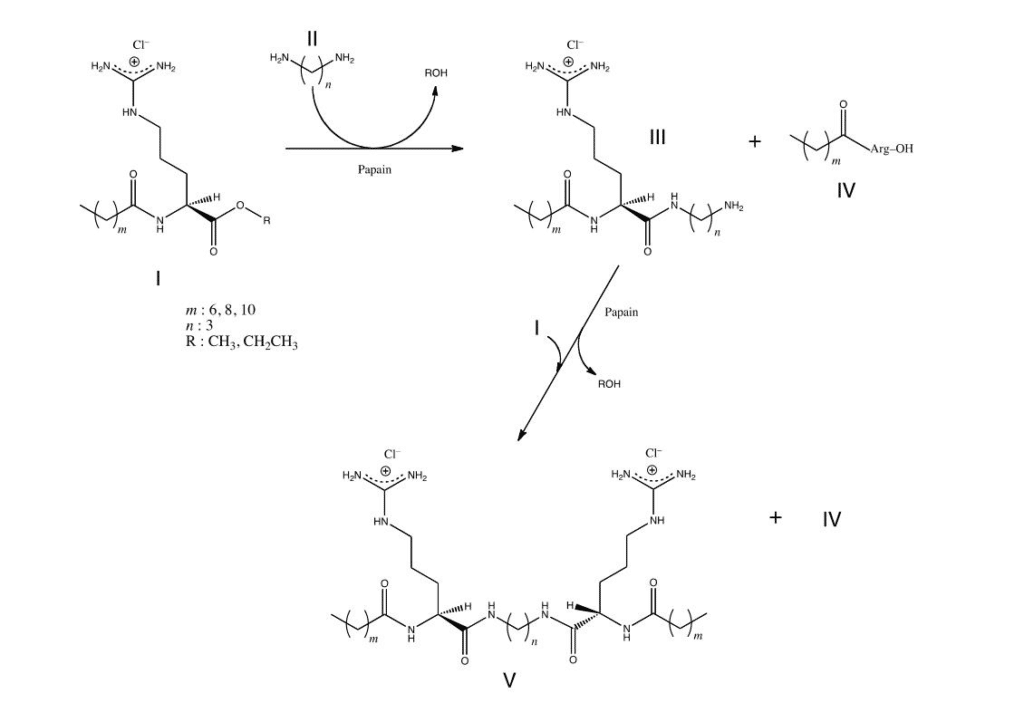
Tissue Engineering
The arginine-glycine-aspartate (RGD) motif has critical impact in the mediating integrin-matrix interaction. RGD and RGD-containing peptides are competitive and reversible inhibitors of the binding of adhesive proteins. These peptides have been applied to study the adhesive interactions between cells, suppress tumor metastasis and platelet aggregation.
Conclusion
Chemoenzymatic peptide synthesis has higher yields and lower toxic chemicals, it is an excellent alternative to conventional chemical peptide synthesis. However, there are some certain drawbacks need to be addressed, such as difficult sequences controlling and longer chains synthesis. The combination of engineered proteases, substrates mimetics, and reaction media can overcome the present issues. Qyaobio has advanced CEPS technology in long sequence and cyclic peptide synthesis.