Click Chemistry in Peptide Synthesis
Introduction
Click chemistry can generate substances by joining small azide and alkyne units together. It is a rapid method to generate structural diversity in peptide synthesis. Click chemistry is a chemo-selective synthetic method for coupling molecular fragments in mild reactions conditions. Since, the click chemistry approach is applied broadly to construct diverse chemo-types in both chemical and biological fields. Peptide synthesis applies click chemistry to form triazoles for different requirements, such as mimicking peptide and disulfide bonds, peptide secondary structure, functional group linkage, bio-conjugation. In order to discovery new synthetic approach to convert peptides with structure-minimized and more stable forms.
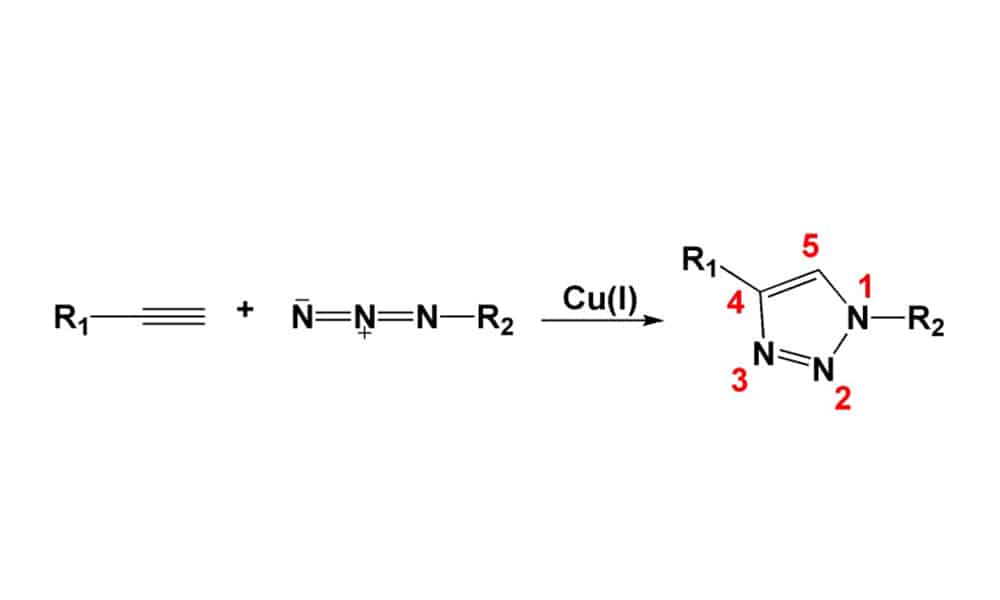
As click reaction is one kind of Huisgen cycloaddition reaction, it forms five-membered hetero-cylces like triazoles. Copper-catalyzed azide-alkyne cycloaddition can perform successfully in polar media, this gives rise to a remarkable escalation in use of Huisgen cycloadditions. Recently, new click chemistry are applied in various biological systems, like ruthenium-catalyzed azide-alkyne cycloaddition [14] and copper-free click chemistry.
Alternative for Unstable Bonds
The 1,2,3- triazoles are alternative for unstable bonds in custom peptide synthesis. The stability of natural peptides are limited by the natural bonds, which are subjected to proteolysis by various proteases. Such as disulfide bond can stabilize secondary or tertiary structure in peptides and proteins, but unstable in redox or thiol/disulfide exchange conditions. The replacement of unstable bonds with triazoles can not only maintain biological activity, but also improve the drugability of peptides.
Bioisostere of Amide Bond
The 1,2,3-triazole is the biosiostere of the amide bond moiety of peptides. These two moieties have similar physico-chemical properties in size, dipole moment, and H-bond acceptor capacities. The most critical point is 1,2,3-triazoles are extremely stable to hydrolysis. Triazoles are excellent mimics of peptide bond group, these moieties will retain native-like structure of modified peptides without compromising biological activity.
Substitution of Disulfide Bond
The 1,5-disubstituted triazoles can replace a dislufide bridge in the mono-cyclic variant of sunflower trypsin inhibitor-I (SFTI-1[1,14]), in order to retain nearly full biological activity. In addition, 1,4-disubstituted triazoles can replace two disulfide bonds in tachyplesin I (TP-I). The TP-I is a 17 residue bicyclic peptide with antimicrobial activity, it has the β-hairpin ribbon structure in active form. Finally, the triazole bridged analogue can mimic the secondary structure and release antimicrobial activity.
Replacement of Aromatic Rings and Double Bonds
1,2,3-triazoles are also applied to mimic aromatic rings, acyl-phosphates, and trans-olefinic moieties in peptide synthesis. As triazole is a basic aromatic heterocylic compound, it cannot replace neutral aromatic Phe and Tyr side chains straightforwardly. However, these replacements will enhance binding affinity or bio-activity in some research cases.
Stabilize Functional Structures
Natural peptides have flexible conformations, this limits the ability of achieving the specific and robust action mode. Chemical peptide modifications like secondary structure mimicry or cyclization will increase the structural rigidity and restrict the structure into bio-active conformation. 1,2,3-triazoles are applied to mimic structures of β-turn, cis/trans conformation, α-helix, cyclization.
β-turn Surrogate
Cu(I)-catalyzed alkyne-azide(CuCAA) cyclo-addition is an efficient convergent strategy for β-turn mimicking, the 1,4-connected 1,2,3-triazole ring will provide the similar geometry of β-turn. Therefore, the cyclo-addition between peptides with azides and terminal alkynes can synthesize β-turn units. Moreover, the tendency of β-turn formation in the triazole system depend strongly on the linker length. A three-carbon linker in optimal for stable β-turn formation.
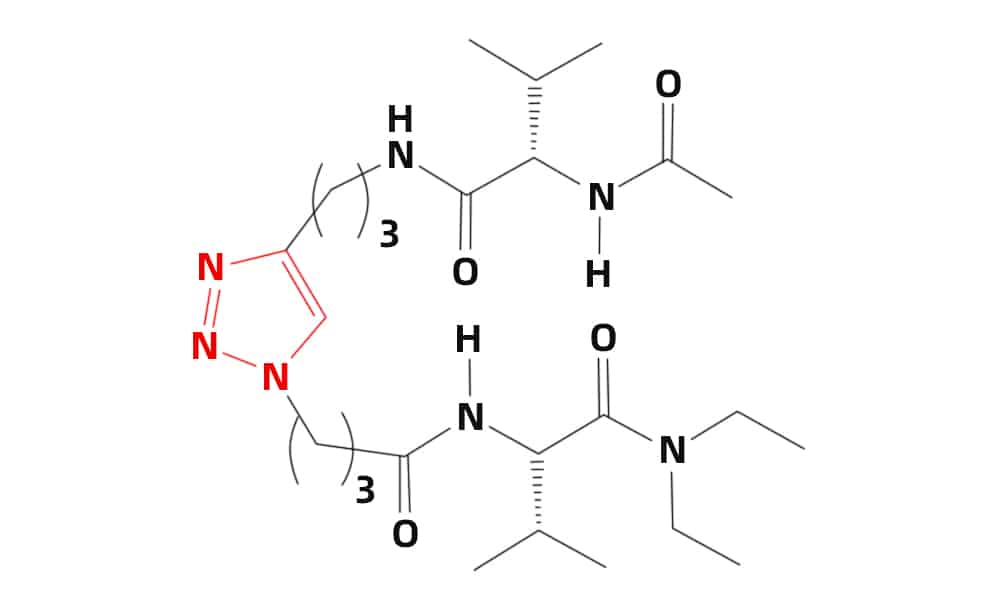
Furthermore, the A 1,4-disubstituted 1,2,3-triazole is a surrogate for the trans amide bond, in order to create the library of 16 diastereomeric pseudotetrapeptides as β-turn mimetics. All these compounds adopt distinct, rigid, conformational structure.
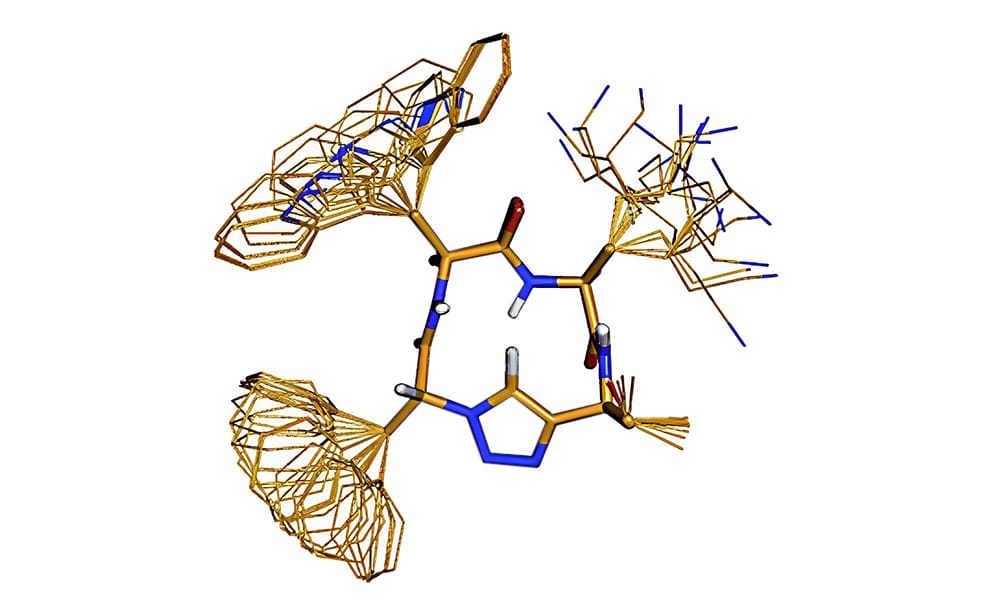
Cis/Trans Conformation
1,4 or 1,5-disubstituted 1,2,3-triazoles can mimic trans and cis amide bonds respectively as selective HDAC inhibitors. The structural and functional properties result in subtle conformational differences, and finally affect the HDAC inhibitory activities.
In addition, the cis/trans ratio of prolyl energy levels have the crucial role in various biological process. The cis-percentage and the conformational stability have coordinated adjustability toward intramolecualr H-bonding effects.
α-Helical Structure
Click reaction can cyclize the linear peptides with intra-molecular side-chain to side chain, in order to achieve rigid confirmations, such as: specific ensemble of bio-active conformation, reduced susceptibility toward proteolytic enzymes. Thus increasing the metabolic stability in vitro and more significantly in vivo. Structural studies indicate that the 1,2,3-triazolyl can accommodate nicely with α-helical structure and reproduce the helical structure by a lactam bridge.
Peptide Cyclization
Head-to-tail peptide cyclization is a critical method to increase structural rigidity and stability of peptides, exoproteases like aminopeptidases or metallo-carboxypeptidases can recognize the N- or C- terminal groups and hydrolyze peptides. Therefore, head-to-tail cyclo-peptides are more proteclytically resistant than the linear counterparts.
Bioconjugation with Different Functions
Click chemistry has exceptional importance in bio-conjugation, in reason of its bio-orthogonal, bio-compatible, chemo-selective and stereo-specific potential. Peptide conjugation can covalently link biomolecular and synthetic building blocks to peptides. Therefore, click chemistry is a powerful tool in peptide conjugation.
Peptide-Carbohydrate Conjugates
Carbohydrates involve in different physiological processes from cell signaling to pathogen defense. Click chemistry is a powerful tool to design and synthesis diverse arrays of peptide-conjugates, in order to satisfy various application requirements, such as vaccines, antibiotics and therapeutics.
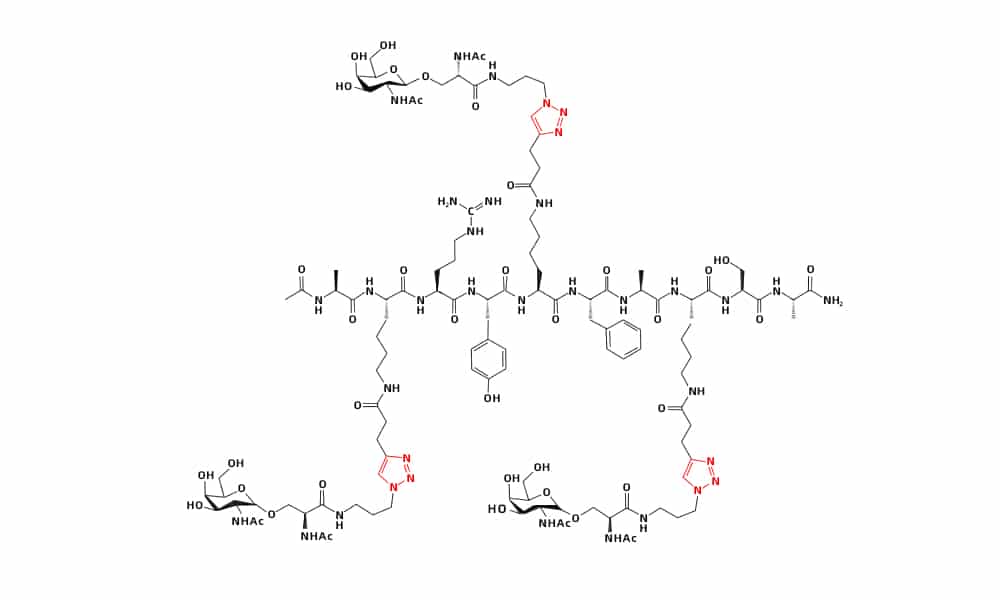
Glycopeptid derivatives are coupled in the click reactions, and display the multitude of biological and pathological functions. Comparing to monomeric peptides, click conjugation of alkynyl dextran with azido peptide has higher stability, tumor targeting and pharmacokinetic properties. Glycosylated cell-penetrating peptides (CPP) can overcome the constraint of non-selective association. Myristoylation and glycosylation of CPP by click chemistry can control the internalization of bio-active peptide to specific cells and enhance the bio-availability.
Conjugation for Drug Delivery
Click chemistry can incorporate different types of functionalities to peptides. Cu(I)-catalyzed 1,3-dipolar cycloaddition can conjugate peptide-based hydrogel to sensitive PEG, in order to improve peptide bio-compatiblity. The peptidodendrimers have the ability to delivery cargo across the cell membrane, lipid bilayer in lipopepitdes demonstrate the function in drug delivery.
Anti-microbial peptides conjugation with pore forming molecules can increase the selectivity and efficiency against biological membranes.
Radio- and Fluorophore-Labeling
Click chemistry normally react in mild conditions with aqueous media, this also results in wide applications in radio-labeling of bio-active peptides. Furthermore, click reactions also have advantages over other labeling technologies in florescent labeling, due to the inertness of chemical reporters and exogenously delivered probes, as well as high selectivity and efficiency of reaction between the reporter and probe. Both copper-free and copper-mediated click reactions are viable tool for sequentially biological conjugation without protecting of the functional groups. Click chemistry is useful for various imaging and tracking application in peptide synthesis.
Enhance Group Affinity
Click chemistry in peptide synthesis can link functional groups to increase structural diversity and improve bio-activity. The substituted alkynes with diverse aromatic, aliphatic, acidic, or basic features, are applied to identify structural characteristics. Then provide high binding affinity in peptide synthesis.
Conclusion
Click chemistry is applied widely in custom peptide synthesis. Because of the structural similarity, it is a surrogate of amide and disulfide bonds. This is very for custom peptide synthesis with specific research purpose.
Qyaobio has excellent peptide synthesis experience in click chemistry, contact us now for your specific peptides in research.