Cyclic Peptide
Cyclic peptides have unique structure in 2D&3D formation.
Cyclic peptides (CPs) are studies widely over decades, this class of peptides are applied in different fields, such as pharmaceutical industry, agricultural applications, diagnostics and vaccines. Cyclic peptides are both discovered in nature and synthesized in laboratory. The cyclic peptides have length ranges from just two to hundreds of amino acid residues. Cyclic peptides are frequently antimicrobial or toxic in nature, while applications as antibiotics and immunosuppressive agents in medicine.
QYAOBIO has solid expertise in manufacturing of conformationally constrained peptides. Peptide cyclization is a frequent strategy for mimicry of secondary structure, or creation of conformational stability. In addition, Thin-Layer-Chromatography (TLC) is a convenient method to detect cyclic peptides.
Cyclic Peptide Classifications
Ring Bond Formation
Cyclic peptides are normally classified according to the bond types of the ring formation.
Homodetic cyclic peptides
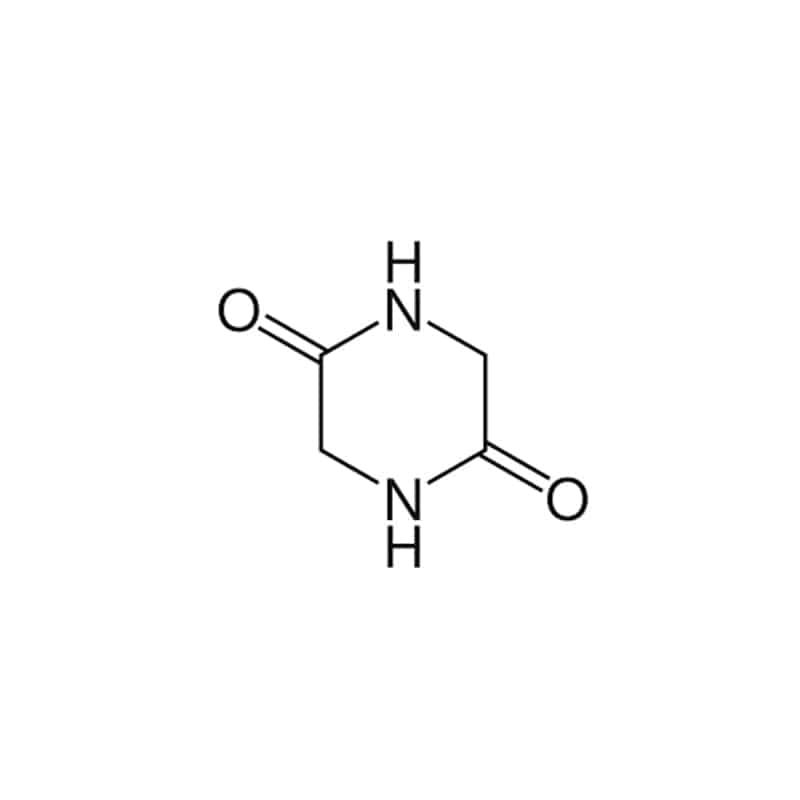
These kinds of cyclic peptides are composed of normal peptides bonds in the ring, such as the bond between the alpha carboxyl of one residue to the alpha amino of another. The smallest homodetic cyclic peptide is 2,5-diketopiperazines, it is derived from the cyclization of a dipeptide.
Cyclic isopeptides
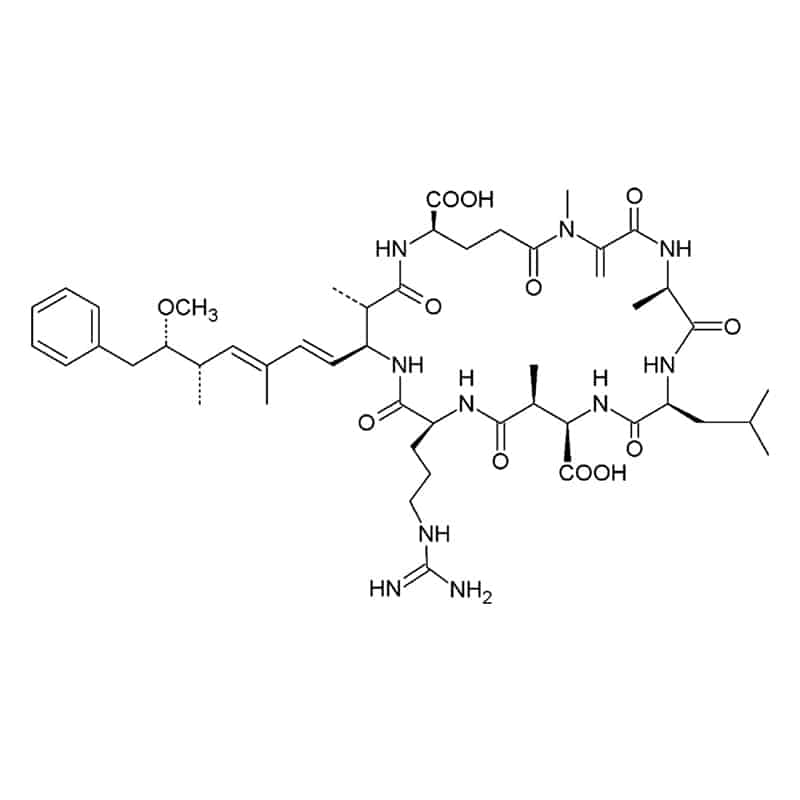
Cyclic isopeptides contain at least one non-alpha amide linkage. Such as in microcystin and bacitracin, there is a linkage between the side chain and the alpha carboxyl group of different residues.
Cyclic depsipeptides
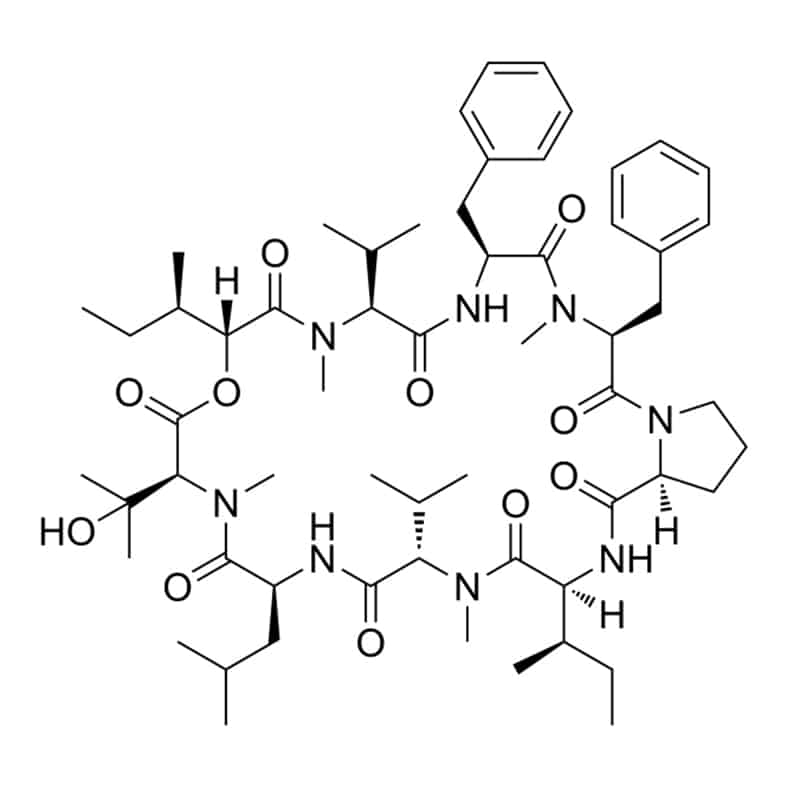
These pepetides contain at least one lactone/ester linkage in place of the amide, like aureobasidin A, HUN-7293. Other cyclic depsipeptides have linkage between the C-terminal carboxyl and the side chain of Thr or Ser residue. Such as kahalalide F, theonellapeptolide, and didemnin B.
Bi-cyclic peptides
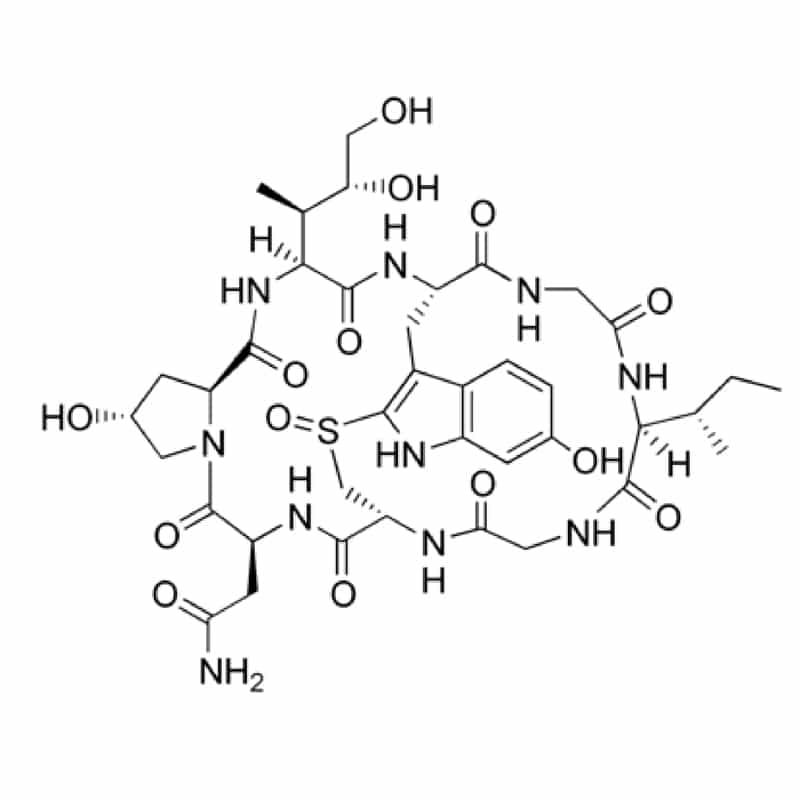
Bi-cyclic peptides contain a bridging group between the two side chains, such as the sulfoxide bridge between Trp and Cys residues in amatoxins.
Disulfide cyclic peptides
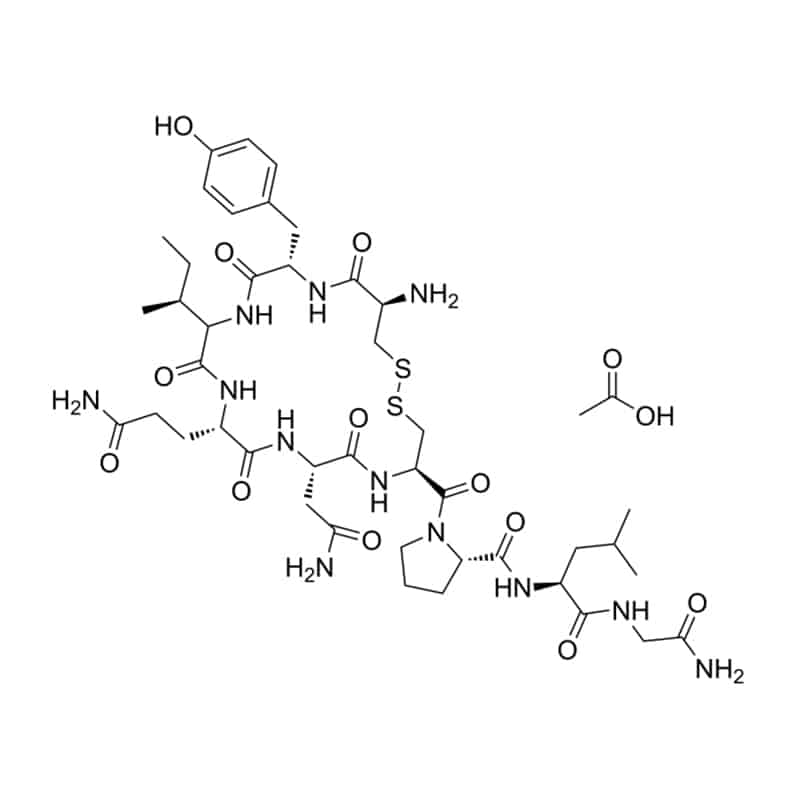
There are some bi and mono-cyclic peptides with a disulfide bond between two cysteines, oxytocin is the notable example.
Cyclic peptides usually contain alternating L and D amino acids, then building blocks stack up to a flat conformational structure. Cyclic peptides provide distinct properties, such as precise diameter control, functionalization through amino acid sequence.
Cyclization Configurations
Cyclic peptide is the specific polypeptide chain with a circular sequence bond. According to the bond position and functional groups in cyclic peptides, these connection bonds have four main categories:
Amino and carbonxyl ends – cyclosporin
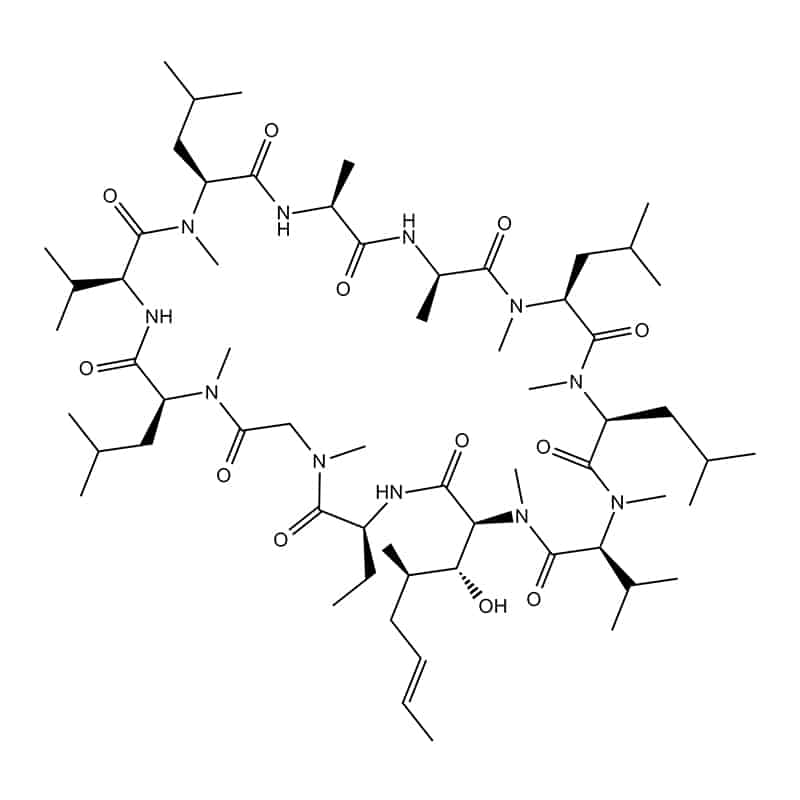
This is the common heat-to-head ligation approach, it occurs between N-terminus to C-terminus. This ligation is formed via amide bond formation of lactamization. The cyclization are most frequently used for confering greater stability.
Amino end and a side chain – bacitracin
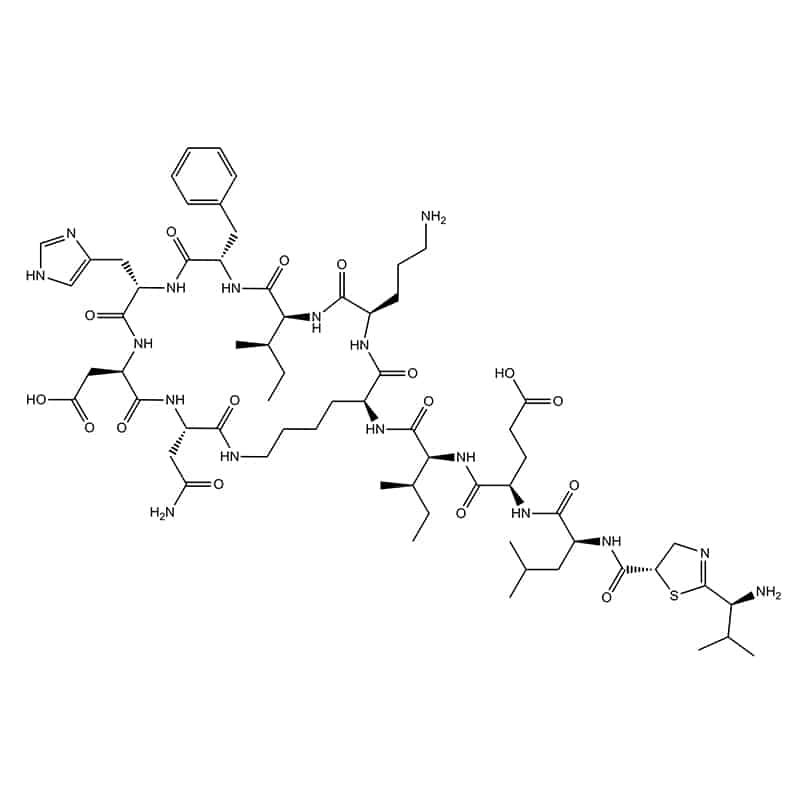
It is also called side chain to head. This ligation occurs between N-terminus and an interal COOH, such as the β-COOH-group of Asp or γ-COOH-group of Glu
Carbonxyl end and as side chain – colistin
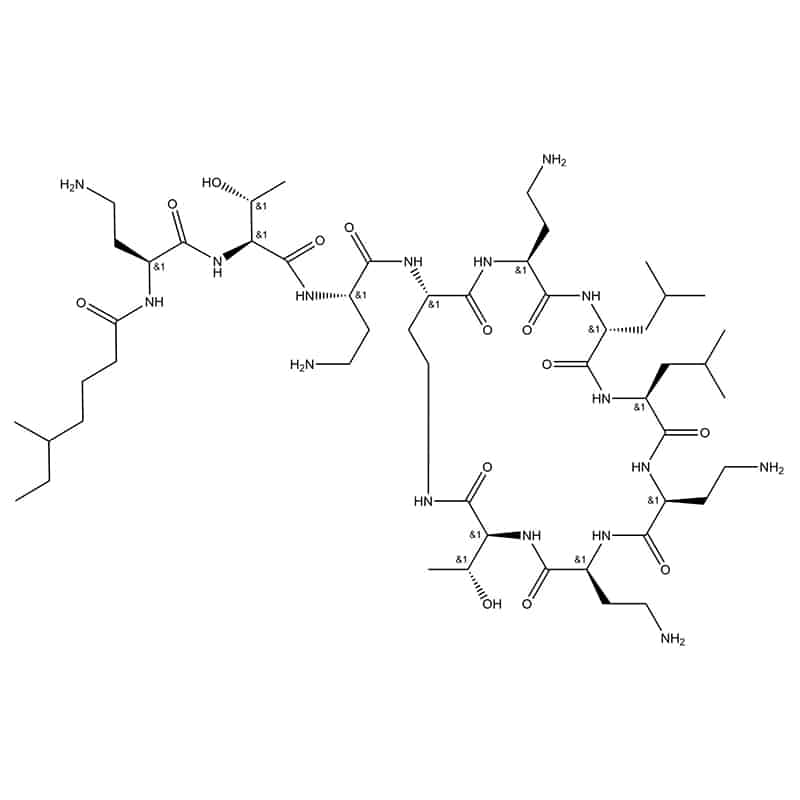
This ligation is side chain to tail, it occurs between internal NH2 groups and C-terminus.
Two side chains/ complicated arrangements – amanitin
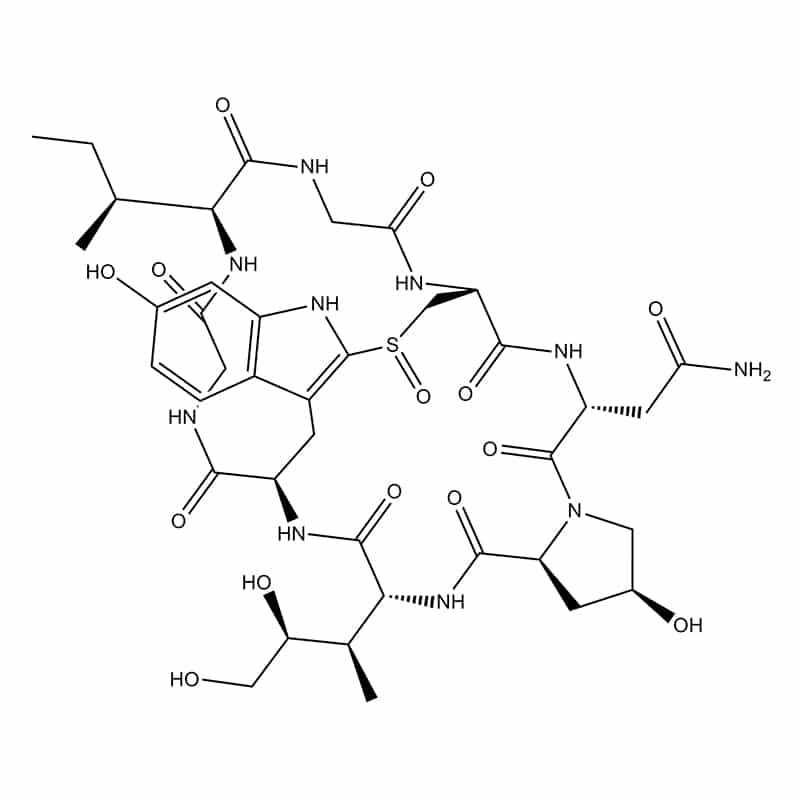
This ligation is also knowing as side chain to side chain, it occurs between side chain of Lysine (ε-NH2–group of Lys) to Aspartic or Glutamic acid (γ-COOH-group).
Peptide Cyclization Strategies
Cyclic peptides are useful for optimization of peptides, such as increase binding potency/selectivity, enhance protease stability. Qyaobio applies the common cyclization strategies as following:
Disulfide (cys-cys) Cyclization
Cys-cys cyclization is the most frequent peptide cyclization method. It is the disulfide (S-S) bond formation between the thiol side chains. The main issue in disulfide cyclization is dimerization. In addition, cys-cys cyclilzed peptides have limited stability of S-S bond, especially under reductive conditions.
Backbone (amide bond) Cyclization
Amide bond cyclization has advantage over disulfide cyclization in chemical stability. Most backbone amide cyclized peptides are synthesized by head-to-tail or side-chain-to-side chain cyclization. The challenges in amide bond cyclization is slow reaction due to entropic reasons. This also results in undesired side reactions like racemization, or peptide capping.
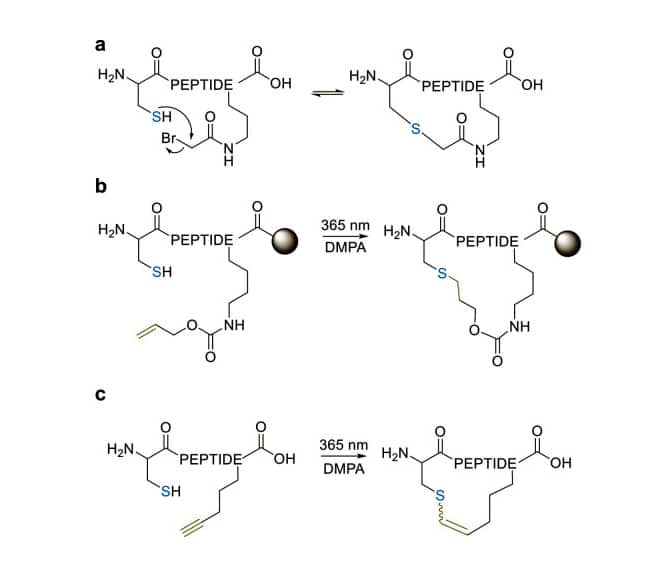
Thio-ether Cyclization
There are three thioether cyclic formation
- Thioether cyclization apply the cysteine thiol to couple with side chains or N-terminus, this method exploit the nucleophilicity of cysteine thiols.
- The thioether bond for cyclization also can be generated by the radical addition of the thiol group to an alkene on a solid support.
- Thiol-yne coupling is another thioether-cyclization method. The alkyne-containing amino acid reacts with cysteine under photo-induction, and synthesize E and Z isomers.
CLIPS (Monocyclic & Bicyclic) Cyclization
CLIPS (Chemical Linkage of Peptide onto Scaffolds) is a proprietary and highly versatile constaining technology. CLIPS technology cyclizes the linear peptides through reaction of thiol-functionalities in cysteines with the small rigid entity. This anchor reacts exclusively with thiols and attaches to peptides via covalent bonds. The CLIPS cyclization technology has unique versatility and easy application. This cyclization reaction is no longer than 30 min at room temperature without any catalysis. The advantage of CLIPS cyclization:
- Applicable for mono and bicyclic peptides
- Cycliztion of stable covalent thio-ether bond formation
- Compatible with unprotected amino acids in side chains
- Custom synthesis by the toolbox in scaffolds with different polarity, rigidity, solubility, functionality, and S-S spanning distance.
Multiple S-S Bonds Cycliztion
Disulfide-rich peptides (DSRs) are a diverse class of bioactive peptides. As DSRs have remarkable chemical stability than linear peptides, these pepetides attract the exponential interest in drug design, therapeutic agents, vaccine development, and cancer immune-therapy. Multiple disulfide bonds enforce the determined conformations close to native state, this is critical for the proper biological expression. Therefore, the connectivity between cysteines is vital, extremely precise chemical synthesis in required to ensure the proper S-S bond.
Oxidative folding is the common method for dealing with cysteine-rich peptides. Although this technique is assumed to synthesize the single themodynamic isomer, it is crucial to ensure the desired SS bond topology. Therefore, an orthogonal protective group strategy is necessary to ensure the formation of a single topological isomer. Qyaobio applies and optimizes different cysteine-protective groups to ensure our quick and straightforward synthesis of DSRs. Such as Mob, Mobm, Mmt, Acm, Ddm, Trt, Dpm, tBu, StBu, Phacm, Msbh, etc.
Therefore, effective peptide cyclization requires thorough expertise with the optimal cyclization reagents and conditions. Over the last decades, Qyaobio has developed deep experience in cyclic peptide synthesis, resulting in high success rates for even challenging peptides.
Call Us
+86(021)-50795728
+86(027)-60707970
