Optimize Peptoid Synthesis
Introduction
Peptoids (N-substituted glycines) are the peptidomimetic molecules for different applications. The automated solid-phase synthesis is the most common approach for selected synthetic peptoids, QYAOBIO can synthesize polypeptoids with various modifications for selected synthetic targets.
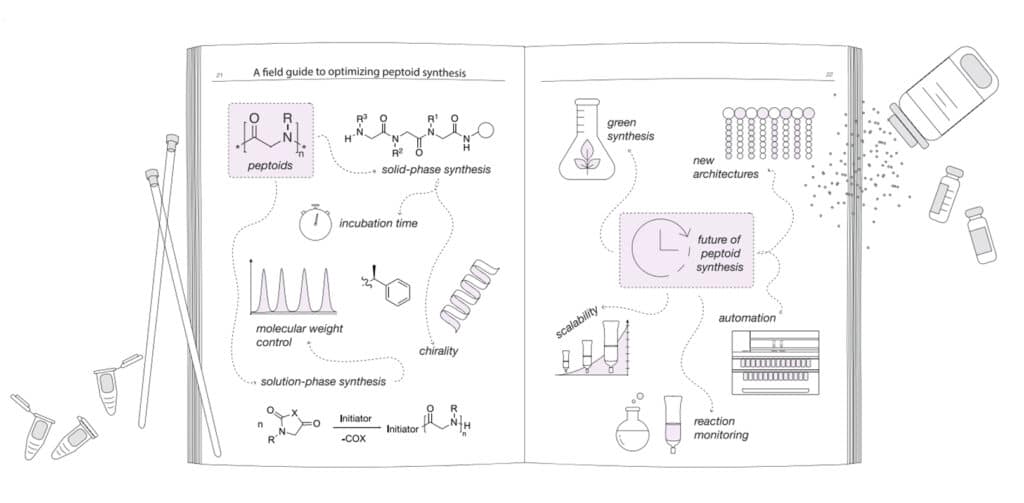
Meanwhile, we also apply new solution-phase synthesis to overcome the limitations in solid-phase synthesis, in order to achieve the high-molecular weight polypeptiods. We have optimized strategies in both solid and solution phase synthesis.
Peptoid Synthesis Strategies
Peptoids have an expanding landscape for therapeutics, diagnostics, carbon capture, and antifreeze agents. The diverse side chains can be incorporated into peptoids, in order to achieve tunable physiochemical properties and secondary structures.
Peptoids have similar bio-compatibility as peptides, but have distinct advantages, such as improved protease stability, increased cellular permeability, and decreased immunogenicity. As advanced synthesis, the wide range of chain lengths can explore the potential utility as both small-molecules and polymers.
There are different strategies for peptoid synthesis
Solid Phase Synthesis
Solid phase synthesis in peptoid provides precise sequence controls, it can be automated in robotic synthesizers. This is limited in milligram scales and oligomers, due to the low effective reaction yields for long chain lengths.
Solution Phase Synthesis
Solution phase synthesis offers the method for polypeptoids with high polymerization (DP) degrees and larger scales.
Optimization of Solid Phase Peptoid Synthesis
The submonomer strategy in solid phase peptoid synthesis is the most common approach, its standard protocols can achieve most instances. While modification can be applied for specific research requirements. We aim to this synthesis optimization, in order to increase reaction efficiency, incorporate different side chains.
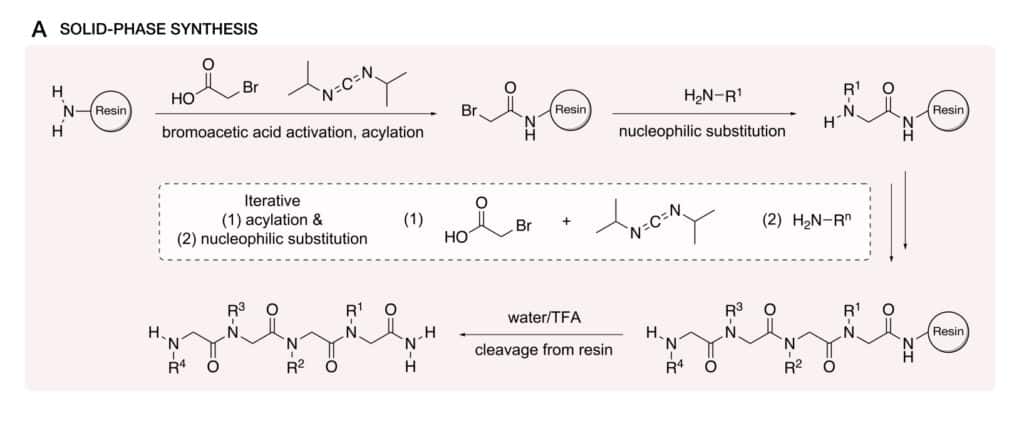
Submonomer Protocols
The submonomer approach involves a two-step cycle: the acylation of the terminal amine bound to the solid resin support, displacement with the primary amine. This strategy has significant benefits for primary amines with diverse side chains, it is more available in synthetic access comparing to the monomer method. In addition, the submonomer method has the modularity and stepwise nature for a wide range of side chains with precise position control in peptoid backbone.
Side Chain Diversity
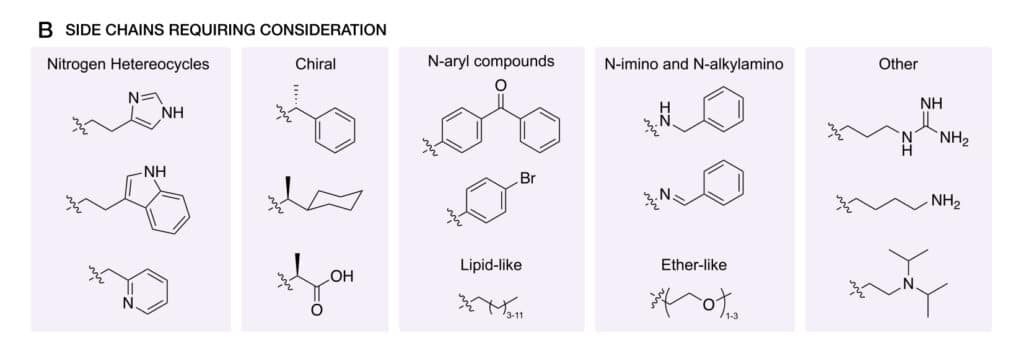
The solid-phase technique provides the opportunities of various side chains, such as self-assembly, biological activity, bio-recognition. In addition, optimization is required for specific side-chains, for example heterocyclic, chiral, bulky, hydrophobic, and charged. In order to prevent cross-section in synthesis and undesired linkages in formation.
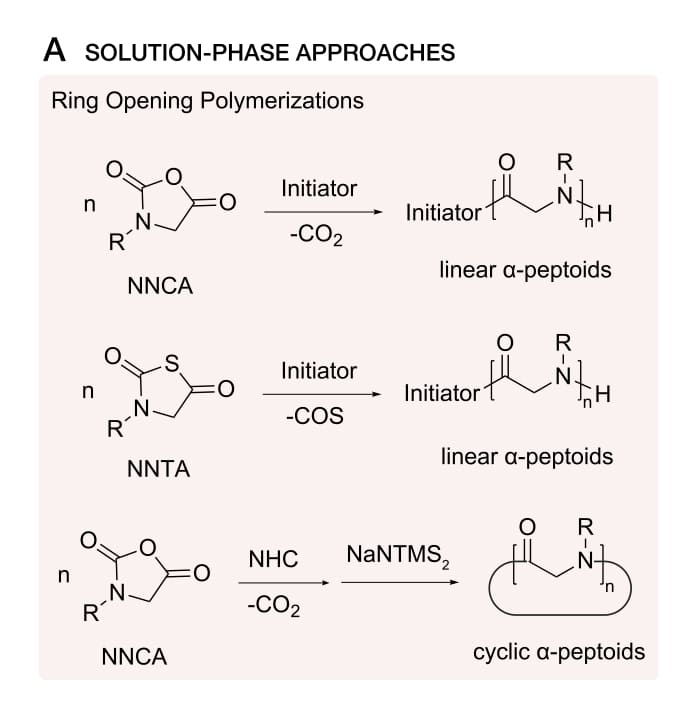
Optimization of Solution Phase Peptoid Synthesis
Solution phase peptoid synthesis with advances increase the side-chain diversity and improve the control of chain lengths. The optimization of solution phase synthesis aim to improve polymerization, solubility, and synthetic versatility.
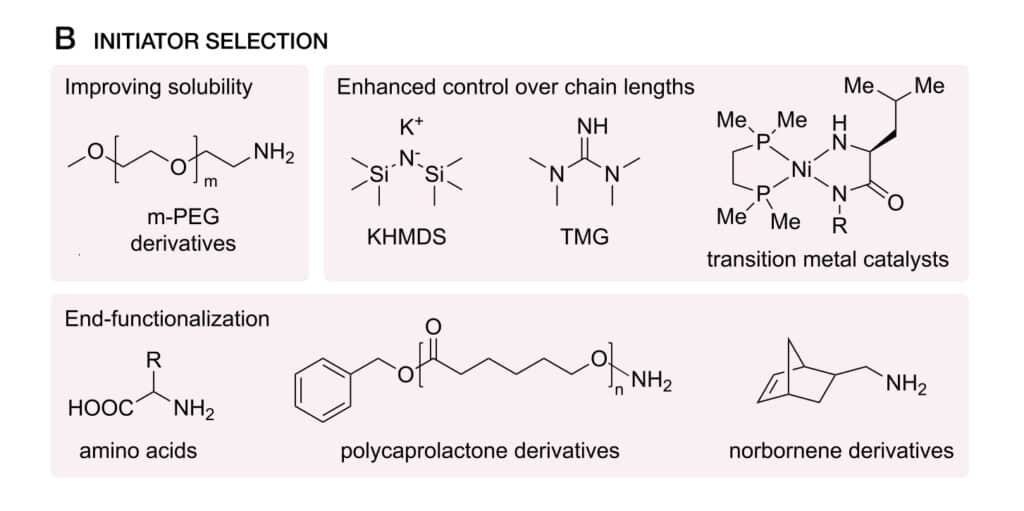
Initiator Selection
The initiator selection will affect the polymerization time, solubility, molecular weight, chain-end fictionalization. Furthermore, initiator will introduce functionality to peptoid chain ends without modification of postpolymerization. It also can influence the control degree in molecular weight.
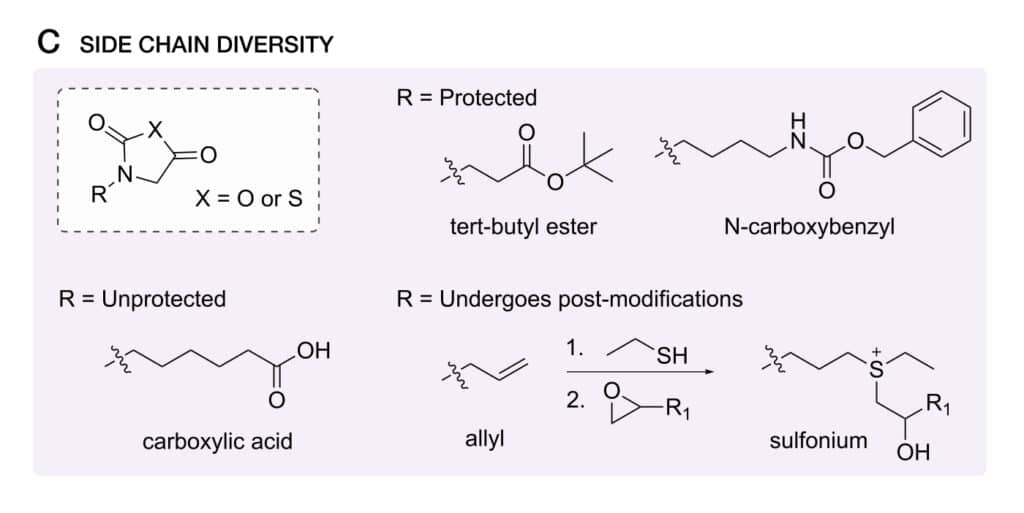
Side Chain Diversity
Once design peptoids for various biomaterials’ application, it is a challenge in synthetic versatility of solution-phase peptoid synthesis. The increased variety of accessible side chains provide opportunities for different application-relevant properties, like self-assembly, biological activity, and bio-recognition.
Polymerization Efficiency and Scale
NNCA(N-substituted N-carboxyanhydrides) monomers and polymers are highly sensitive to air, moisture, heat. And have slow reactivity once apply primary amine as the initiators. Commercial organ-catalysts are applied to increase the speed in solution-phase polypeptoid synthesis. Such as urea derivatives can accelerate the polymerization of bulky NNCA monomers by hydrogen-bonding interactions, and reduce polymerization time from 7 days to 5 hours. Although different strategies are applied to optimize the solution phase of peptoids, the instability of NNCA monomers and high purity requirements still limit the large-scale preparation of polypeptoids.
Peptoid Robotic Synthesis
As the peptoid synthesis reaction in solid-phase sub-monomer method is air and moisture stable. This simple technique is amenable to automation, peptoid synthesis is adapted to most commercial peptide synthesizers. Comparing with manual solid-phase synthesis, automated synthesis involves different concerns and potential drawbacks. Moreover, the significant surface area of tubing in robotic synthesizers require washing between steps, this results in the higher waste volume than manual synthesis.
Different optimization methods are applied for various routes in polypeptoids synthesis, in order to produce peptoids with desired amount and purity. Solid-phase technology can generate sequence and molecular-weight definition in small chain length, it is ideal for peptoid oligomers for drug delivery, therapeutics, nano-materials. Solution-phase synthesis provides higher molecular weights with new initiator and catalyst system, it has excellent control over chain lengths.
The polymerization technology for peptoid synthesis are suitable for different architectures, like: block copolymers, bottlebrush polymers, and star polymers.
Conclusion
Peptoids are a class of biological materials with syntactical access and sequence program. Therefore, the diversity of application result in unique requirements in chain length, sequence definition and control, side chain selection. QYAOBIO anticipate the new peptoid architectures and enhanced synthetic efficiency in peptoids synthesis. We will develop sustainable methods for peptoid synthesis with alternative solvents.