Peptide Biotinylation
Peptide biotinylation can be synthesized at either N- or C- terminus.
The biotinylation of peptides is an efficient method to bind specific peptides to streptavidin coating surfaces. Due to the biotin moiety has strong affinity for streptavidine (Kd<10-10 M). Because of the high affinity and binding specificity of biotin, the biotinylation interaction (biotin-avidin or biotin-streptavidin) is applied as the basis of laboratory research techniques to label, detect and purify peptides.
Peptide biotinylation can be synthesized at either N- or C- terminus. N-terminal biotinylation bound directly to the primary-terminal amino acid, while C-terminal is performed at the ε-amino group of an (extra) C-terminal lysine. Furthermore, we recommend the application of N-terminal modification, because of the low cost, higher success rate, shorter turnaround time, and easy synthetic operation.
Biotinylated Peptide
Biotinylated peptide is designed for biomedical screening assays, which require immobilization onto streptavidin coating surface. The extraordinary interaction of biotin and avidin/streptavidin is applied for various applications in biochemistry. QYAOBIO provides three different biotin labeling.
Biotin
N-terminal
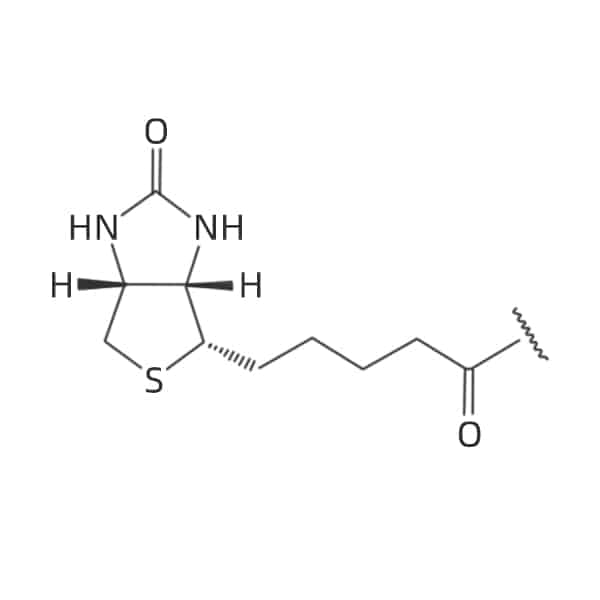
Lys (Biotin)
C-terminal
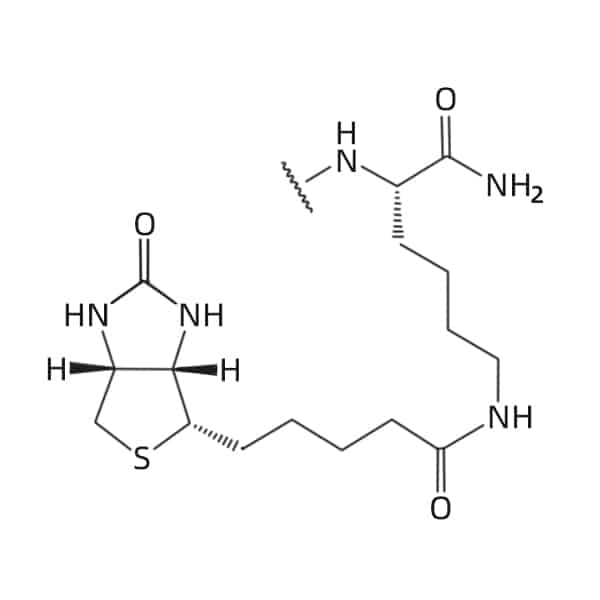
EDA (Biotin)
C-terminal
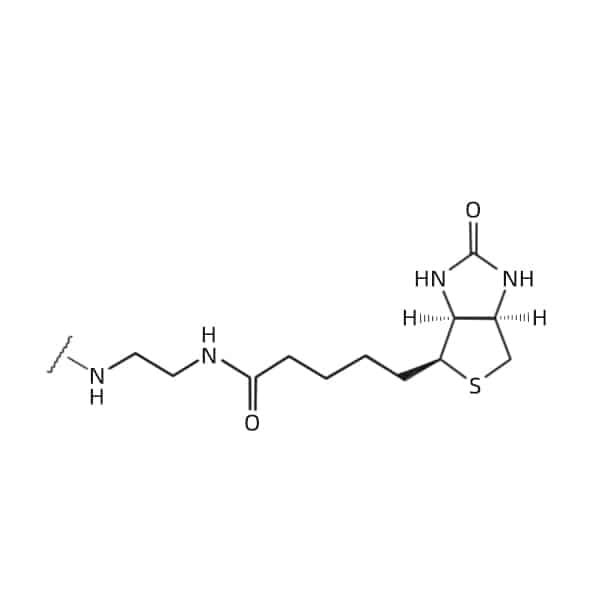
Properties of Biotinylated Peptide
- Each biotin peptide is easily biotinylated at the N-terminus
- The flexible spacer or linker is inserted between biotin and peptide
- Excellent for screening assays like ELISA peptides
- Incorporation of non-natural amino acids, or post-translational modifications like methylation, phosphorylation
Application of Biotinylated Peptide
- The biotinylated peptides are useful tools in immunoassay studies, like connect biotinylated peptides to microtitration plates with antibiotic proteins coating.
- Conjugate biotinylated peptides with avidin magnetic beads to form resins, this is applied in peptide pulldwon test.
- Biotin-avidin or biotin-streptavidin interactions are useful for laboratory research techniques, such as labeling, detecting and purification of peptides.
- Biotin-labeled peptides are detected or purified by avidin conjugates for protein based research applications.
- Identification and optimization of kinase, phosphatase, acetyltransferase, histone deacetylase via standard screening systems.
- Peptide ELISA for mapping of protein interaction sites.
Linker & Spacer in Biotinylation
The critical consideration in peptide biotinylation is the sufficient spacer arm between the biotin group and the amino acids. In order to ensure the expected interaction of peptides and macro-molecules.
The inserted linker or spacer between biotin and peptide sequence can avoid sterical hindrance. We provide multiple spacers and linkers to meet specific requirements, the common spacers or linkers are as following:
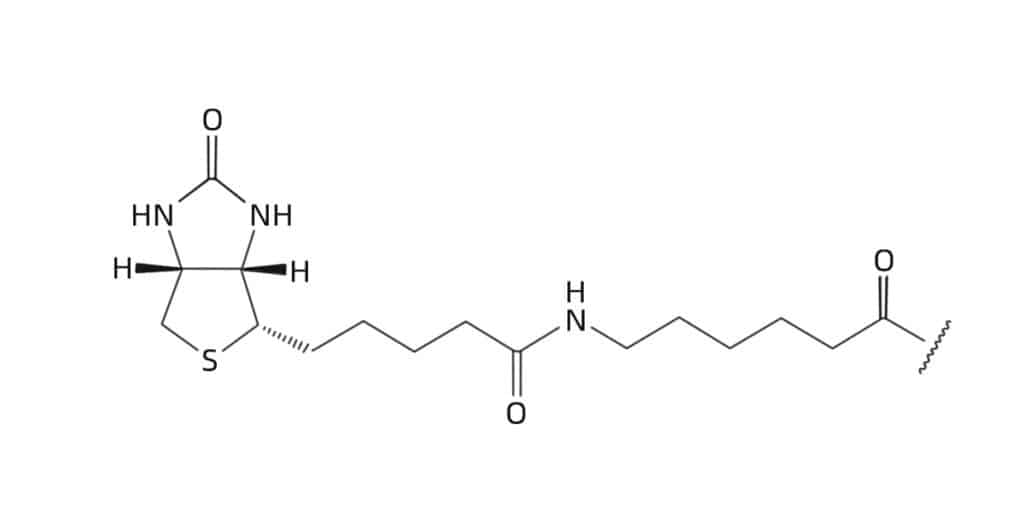
The hydrophobic straight chain spacer like 6-carbon ε-aminohexanoic (Ahx)
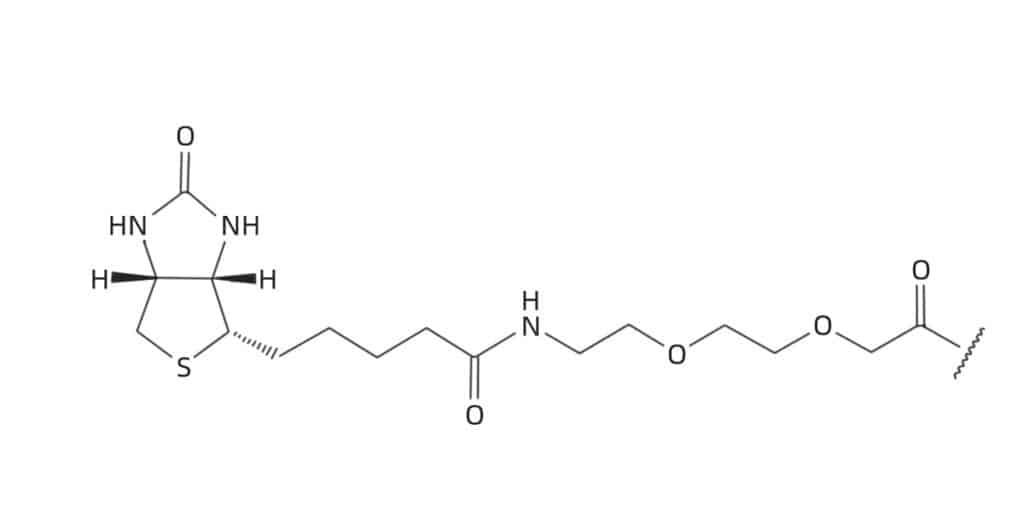
The PEG-spacers of various length
The incorporation of spacer or linker between biotin and peptide is efficient to reduce steric hindrance in the subsequent binding. In addition, the spacer of polyethylene oxide also can improve the solubility of the reagent and biotinylated peptides.
Biotinylation interactions
The critical consideration in peptide biotinylation is the sufficient spacer arm between the biotin group and the amino acids. In order to ensure the expected interaction of peptides and macro-molecules.
The inserted linker or spacer between biotin and peptide sequence can avoid sterical hindrance. We provide multiple spacers and linkers to meet specific requirements, the common spacers or linkers are as following:
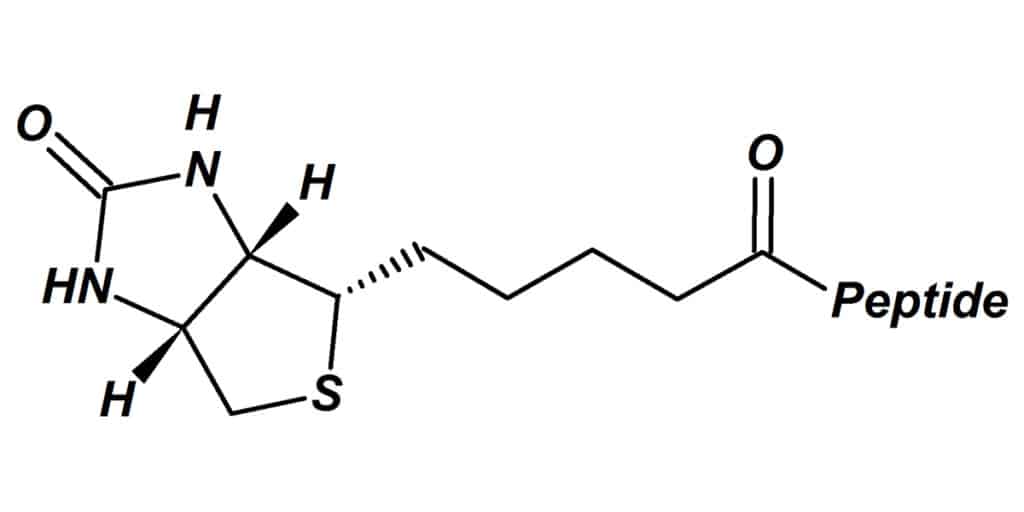
The biotin-avidin (strept) interaction is essentially irreversible in physiological conditions, this is one limitation in purification applications. Biotin binding is resistant to PH or temperature changes, organic solutions and denaturing reagents. Therefore, biotin analogs are recommended once the reversible interaction is required.
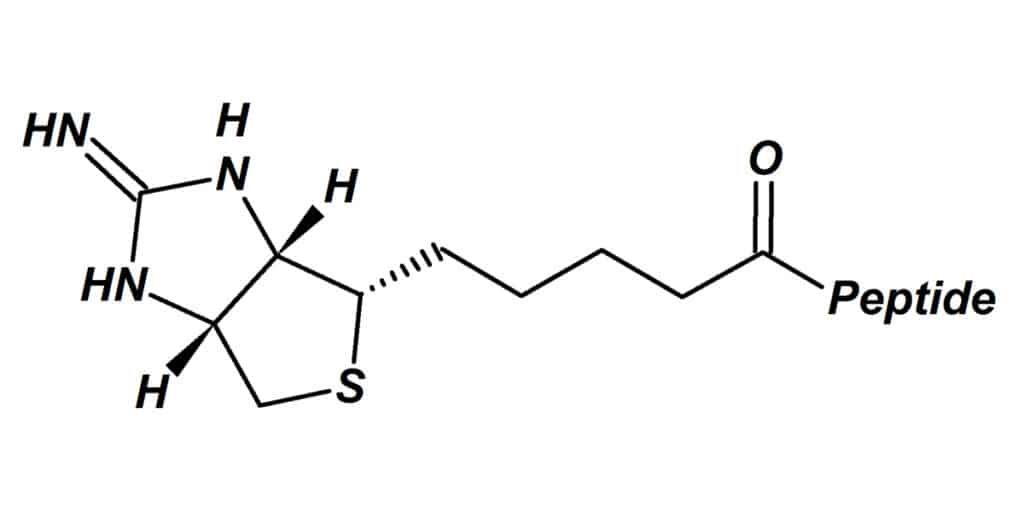
Iminobiotin is an analog of biotin, the upper ring structure is replaced by a 2-imino-imidazole structure. This results in moderated interaction with the avidin or streptavidin binding sites. Therefore, biotin analog can be applied in situations with mild dissociation of avidin (strept) – biotin complex.
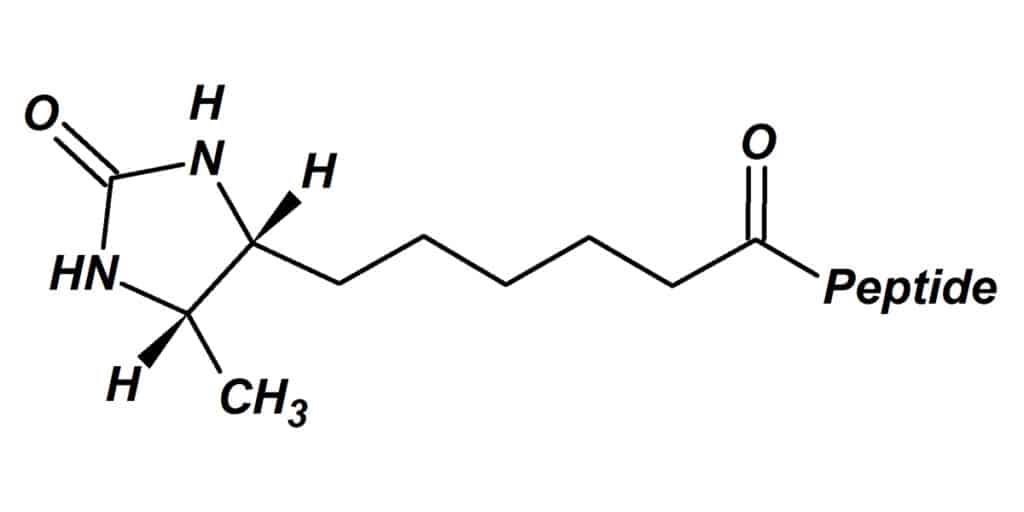
Desthiobiotin is a non-sulfur containing biotin analog, it has less tight bind to avidin and streptavidin than biotin. But it still provides a high level of biological specificity. Moreover, it is less prone to oxidation.
In Fmoc solid phase peptide synthesis, biotin can link to the N-terminus of peptides, or side chains of lysine (Lys) and glutamate (Glu).
Call Us
+86(021)-50795728
+86(027)-60707970
