Peptoid
Peptoid is the common class of peptidomimetic molecules.
Peptoid (N-Substituted glycines) is a class of peptidomimetic molecules, it is the materials for health, environmental, and drug delivery applications. Solid-phase synthesis is the most common method for peptoid synthesis with selected synthetic targets. Meanwhile, solution-phase synthesis leverage to overcome limitations in solid-phase, and achieve high-molecular weight polypeptoids.
Peptide drugs are a rapidly developing class of therapeutics. However, it is hampered by the inherent characteristics: low stability, low target specificity, low hydrophobicity, lack of specific transportation systems. Therefore, peptiod synthesis can overcome these peptide disadvantages and satisfy the increasing requirements in drug target discovery and lead structure discovery researches.
Peptide vs Peptiod
Structure comparison
Peptoids (N-Substituted glycines) are peptidomimetics with side chains appending to the nitrogen atom in the peptide backbone. In native peptides, the R groups are 20 different substitutions for specific amino acids. While in peptoids, the R group selections are much wider without potential limitation. Notably, peptoids are lack of amide hydrogen, which is responsible for secondary structure elements in peptides.
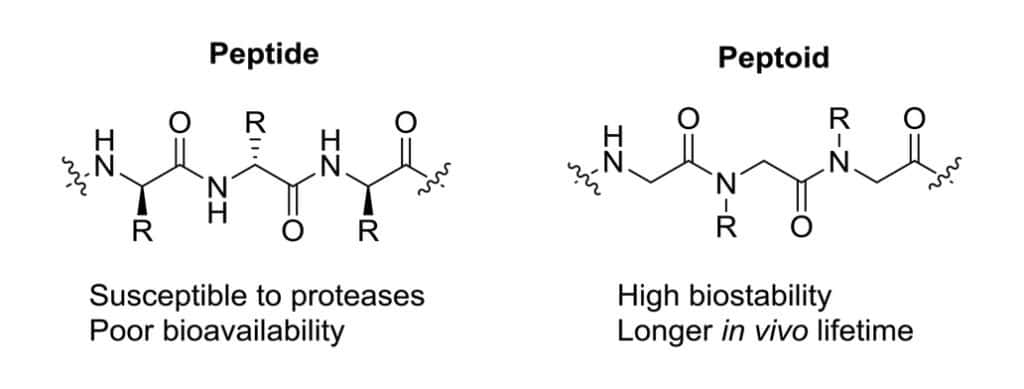
In reason of the availability of synthetic building blocks in peptoids, the peptoid-base therapeutic development have a wider range of modular diversity. Comparing to traditional α and β peptides, peptoid-peptides provide distinct conformational space and diversity.
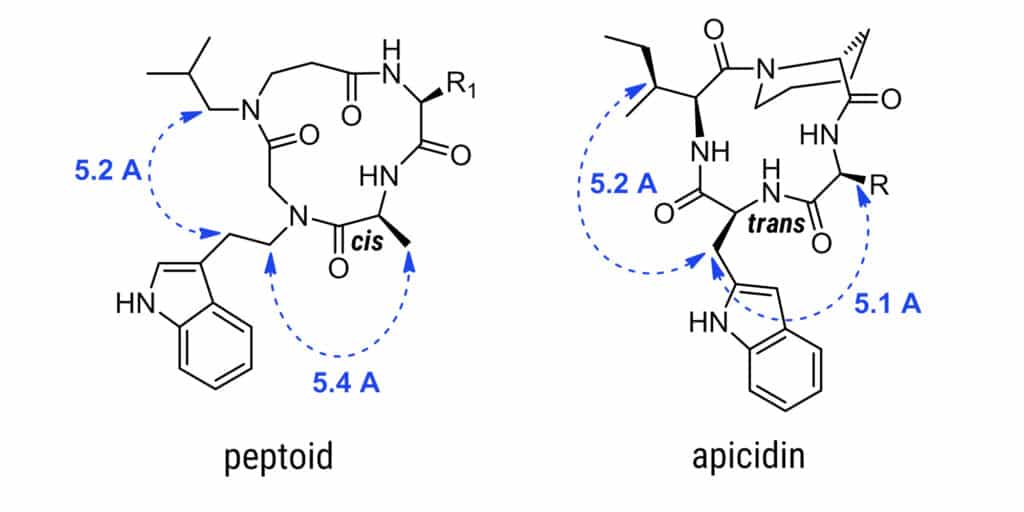
Such as: Ampocidin as a HDAC inhibitor is a therapuetic compound for anticancer chemotherapy. Comparing the peptiod-peptide in the right, although the different cis-trans configurations of amide bonds, the distance between pharmacophoric side chains for molecular recognition are almost identical. Peptoids have advantages over small molecule drugs because of receptor selectivity, potency, and decreased off-target activity.
Characteristic comparison
Peptoids have several advantages over peptides:
- High stability: Peptoids are less susceptible to degradation than peptides in vivo.
- More diversity: Peptoids provides more combination approaches in drug discovery, because the large libraries is synthesized easily from available primary amines.
- Cost-efficiency: It is inexpensive and quick to mine half millions of peptoids in advance screening methodology. In order to satisfy further highly specific protein-binding.
- High potential marketing: peptoids has great potential for pharmacological agents.
Peptoid synthesis service
Peptoid Synthesis Method
Peptoid synthesis in Qyaobio can incorportae peptoid and peptide residues into one sequence with unique backbone atoms between side chains. The ability of varying side chains distance and extensive side-chain diversity encourage the peptoid modulation with enhanced binding affinities, lower cytotoxicity, better solubility, and increased cell-permeability. Except the changes in backbone periodicity, the peptiod residues can expand into the cis-conformations, which is absent in the typical trans amide bonds of peptides. The peptoid synthesis methods are solid-phase, side reaction, monomer and submonomer.
Solid-Phase synthesis
The first oligopeptoids are synthesized by solid-phase synthesis with a set of Fmoc-protected peptiod monomers. The main limitation of this method is that it requires extensive synthetic preparation of chemically diverse monomers. In addition, the secondary N-terminal amine in peptoid has more sterical hindering than the primary amine, this slows down the coupling reactions.
Side Reactions
There are some competing side reactions for unique peptoid synthesis, such as peptiod dimer synthesis, normally lead to the formation of cyclic diketopiperazines. Sub-monomer, with nucleophile atoms from the amino nitrogen in side chains, are prone to cyclize after bromoacetylation.
Monomer peptoid synthesis
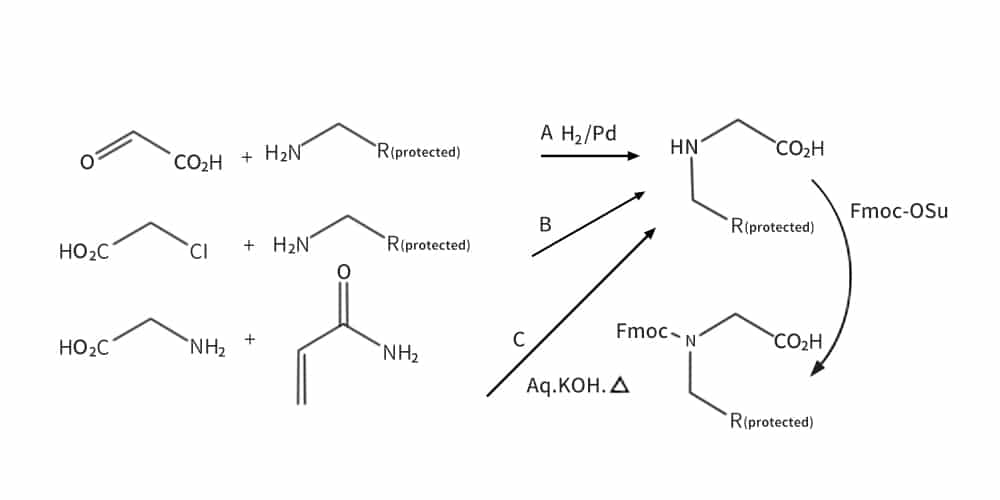
Monomer peptoid synthesis is similar to solid-phase peptide synthesis, except all protected monomers for peptoid synthesis should be synthesized firstly. The synthesis of every Nα-protected monomer is tedious and time-consuming.
Submonomer peptoid synthesis
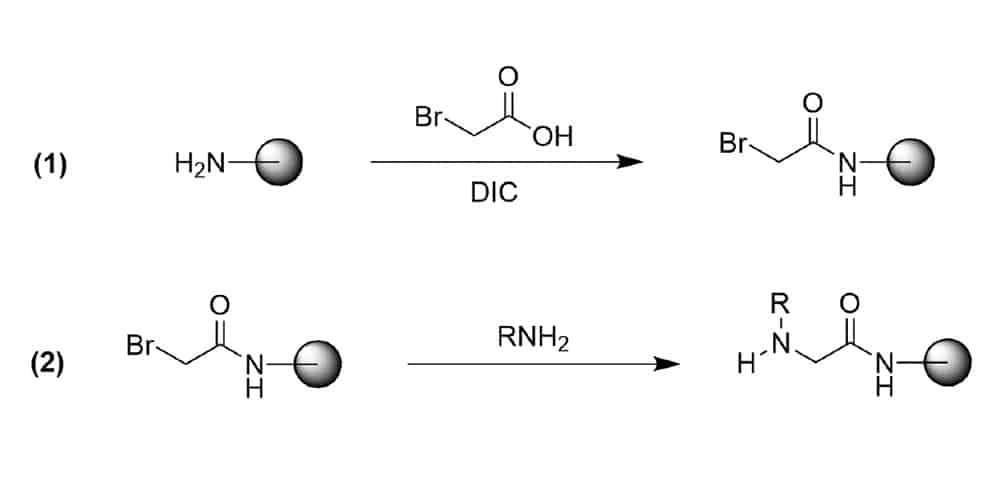
Submonomer method eliminate the requirement of Nα-protected monomer. Each residue is installed in two steps: acylation then nucleophilic displacement. In acylation step, the haloacetic acid (bromoacetic acid typically) is activated by diisopropylcarbodiimide to react with the amide of the previous residue. Then the amine displaces the bromide to form an N-substituted glycine residue in the displacement step. This method has advantages over the monomer approach, and is applied widely to peptoid library construction.
Peptoid Applications
As peptide-based neurotherapeutics provide high specificity and tolerabilty, but limit by proteolytic degradation in vivo. Peptoides, or N-substituted glycines are attractive and novel neurotherapeutic candidates. Thess peptoids demonstrate higher cellular permeability and less immunogenicity than corresponding peptides. In addition, peptiods are less likely to elicit the immunogenic response.
Aggregation inhibitors
The inhibition of Aβ aggregation by peptiods are widely studied in neurodegenerative amyloidogenic proteins. Each peptoids inhibitors have aromatic side chains, this indicates the importance of structural element in recognition and disruption processes.
Neuroprotective Agents
Peptoids protect against neuronal loss by targeting the apoptotic signaling pathways, in order to avoid the deleterious side effects.
Cell signaling effectors
The cell signaling intervention and modulation is an additional method for CNS therapeutics. Peptoids are effective modulators of cell signaling within CNS, and have potential capacity in therapies of neurodegenerative diseases.
Neuroinflammation Attenuation
The suppression of neuroinflammation is a target for therapeutic. Peptoids amplify weak immune response or weaken deleterious immune response by functions as antigen surrogates throngh competition. In order to attenuate neurodegeneration-associated inflammation by a variety of mechanisms.
Biomarker Detection and Imaging
Peptoids are bimimetic receptors with diagnostic applications through their specificity and stability, relatively low cost, and ease of modification. The combinatorial library of peptoids can provide random and diverse molecules for the capture agent to detect the biomarker.
Call Us
+86(021)-50795728
+86(027)-60707970
