Hydrophobic Interaction Chromatography
QYAOBIO provides hydrophobic interaction chromatography for customers in worldwide
Hydrophobic interaction chromatography (HIC) can separate molecules according to the hydrophobicity. HIC is a useful technique for protein purification with maintenance of biological activity, it normally applies less denaturing conditions.
HIC Steps
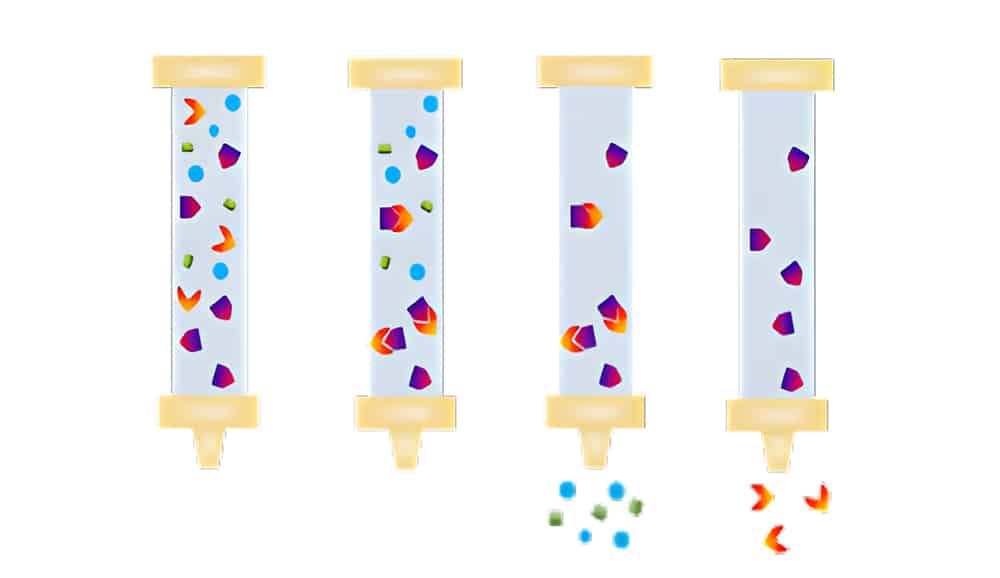
There are four main steps in bind/elute mode of HIC:
Equilibration
Prepare the desired column in starting conditions, then equilibrate HIC resin with high-salt start buffer.
Sample Application
Target proteins bind to the ligand on resins by hydrophobic interactions. The sample buffers have the same pH and ionic strength as the starting buffer.
Elution
Decrease salt content in the linear gradient to elute the hydrophobic proteins. The least hydrophobic proteins will elute firstly.
Regeneration
Remove all molecules binding to the stationary phase, ensure the full capacity of resins for the next run.
Theories in Hydrophobic Interaction Chromatography
There are three major theories in HIC mechanism: salting out theory, thermodynamic theory, surface tension theory.
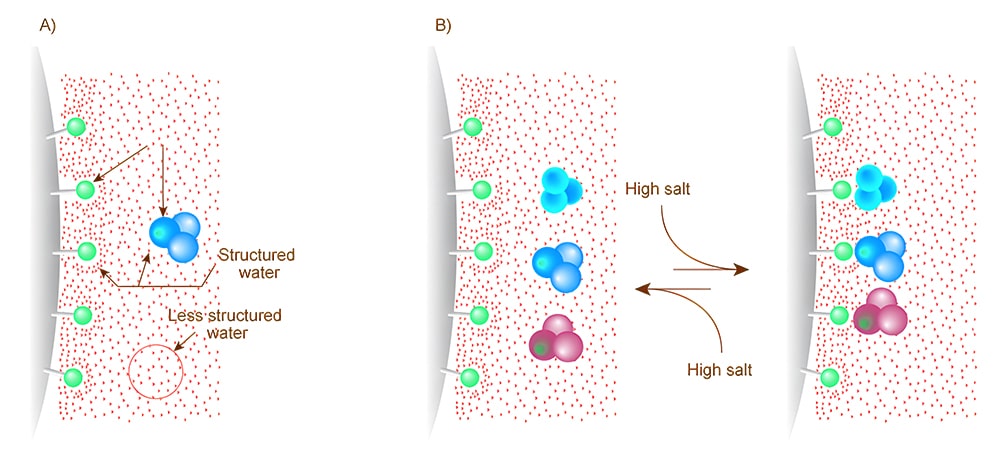
Salting-Out Theory
In salting out theory, the hydrophobic amino acids will fold into protected hydrophobic areas in aqueous solution, while hydrophilic amino acids allow proteins bonding with the hydrogen molecules. Therefore, once there are enough hydrophilic areas in the surface of proteins, the water dissolve probability of proteins will increase.
However, once add the anti-chaotrophic salts (ammonium sulfate, sodium sulfate) in the solution, some water molecules interact with the salt ion, not the charged protein parts. If the protein-protein interactions become stronger than the solvent-solute interactions, the protein molecules react freely with others, then aggregate and eventually precipitate out of the solution. In this case, different addition of salt in the solution will reduce the solubility of different proteins to varying extents.
Thermodynamics Theory
When non-polar molecules contact with the polar solvent like water, there are increased degree of solvent molecules order surrounding the hydrophobic molecule. Therefore, the dissolution of the non-polar molecules in polar solvents cannot occur spontaneously.
However, once two or more non-polar molecules are in the polar environment, the hydrophobic surfaces of macro-molecules will hide from the polar surrounding, then the hydrophobic molecules will aggregate spontaneously.
Surface Tension Theory
The hydrophobic interaction in HIC is dependent on surface tension theory between proteins and immobilized ligands. These forces (van der Waals forces) will increase as the ordered structure of water increases in salting-out conditions.
HIC Applications
Hydrophobic interaction chromatography (HIC) is a versatile technology for capturing, intermediate purification, or polishing. HIC provides the selectivity, which is unavailable with affinity or IEX resins. It is suitable for purification of a wide range of molecules.
- Recombinant proteins and peptides
- Monoclonal antibodies, bi-specific Mbs, antibody-drug conjugates (ADC)
- mRNA
- Plasmids
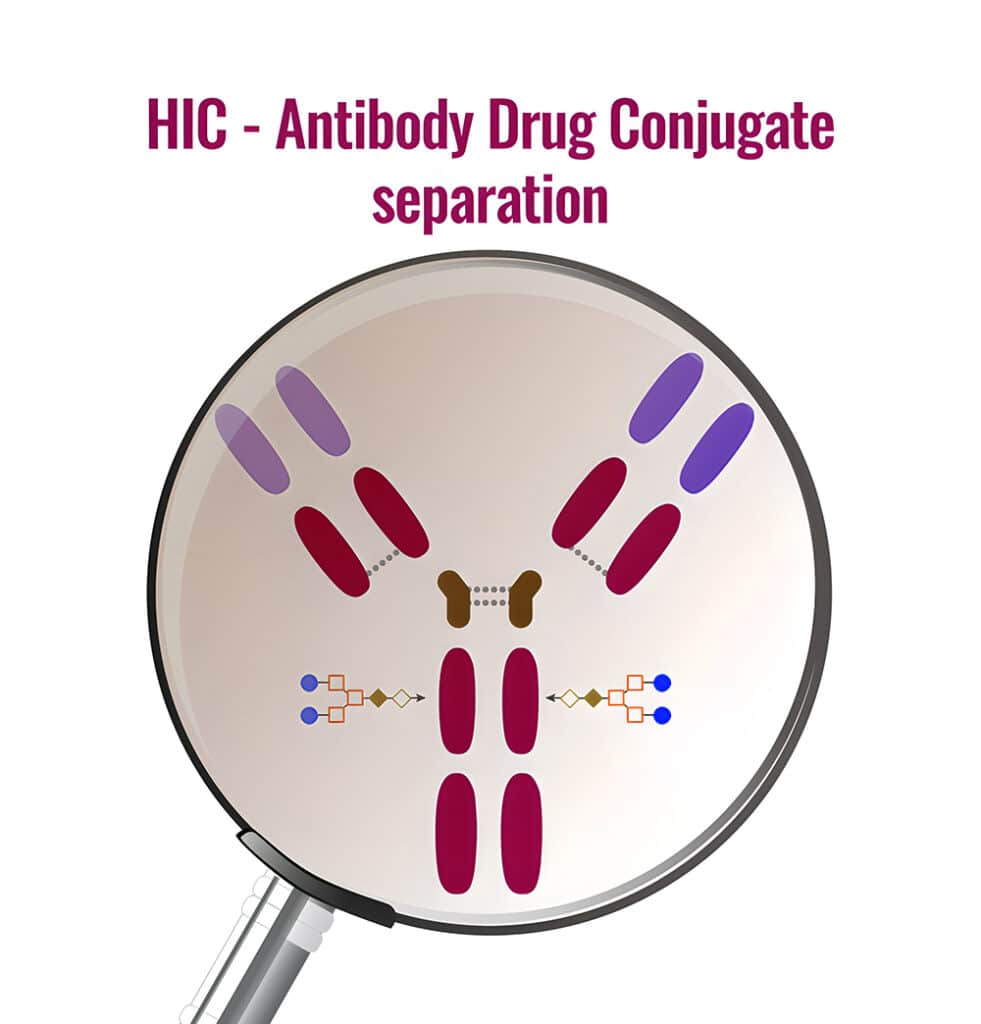
HIC is a valuable tool for protein purification, it is applied in protein purification over a wide range in both analytical and preparatory applications. It also can remove various impurities in the solution.
General Considerations
Hydrophobic interaction chromatography (HIC) is the most popular methods for separating and purifying proteins. HIC is quire useful in isolating protein complexes and in studying protein folding and unfolding. There are different factors influence the results in hydrophobic interaction chromatography, like ligand, matrix, salt concentration, pH, temperature.
HIC Principle
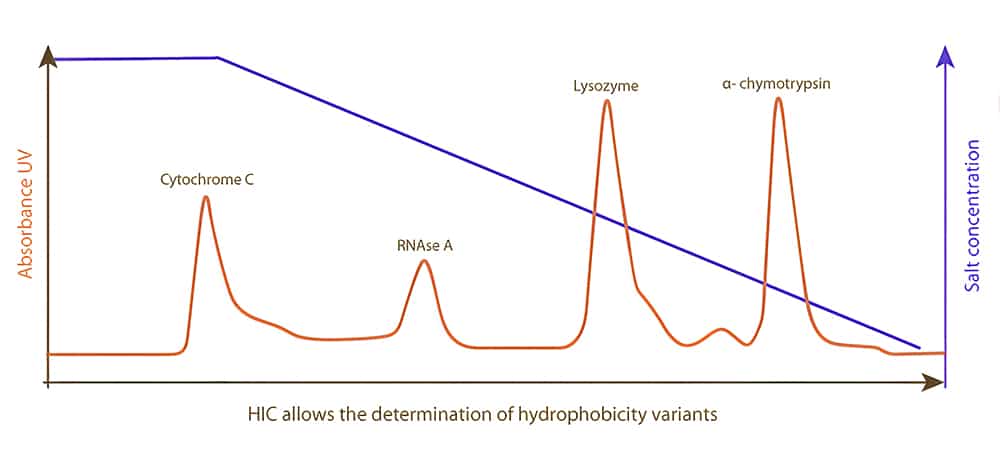
The principle of protein adsorption in HIC is complementary to ion exchange and size exclusion chromatography. Proteins with different degrees of surface hydro-phobicity are seperated by HIC, the proteins bind to hydrophobic ligand in buffers with high salt concentration. Once the ionic strength reduce, the interaction is reversed. Finally, the protein with the lowest hydrophobicity is eluted firstly, the most hydrophobic protein elutes lastly.
Ligand
The type of immobilized ligands determine the adsorption behavior of proteins. Normally, straight chain alkyl ligands show hydrophobic character, while aryl ligands demonstrate mixed mode behavior with both aromatic and hydrophobic interactions. In actual purification, the selection of ligand types is empirically determined.
Substitution Degree
The protein binding capacity will increase with the improved substitution degree of the immobilized ligand. The high level of ligand substitution remain the binding capacity constantly, and increase the affinity of interaction. However, bound proteins in these conditions are difficult to elute.
Matrix
The most common support in HIC are hydrophilic carbohydrates, cross-linked agarose and synthetic copolymer materials. Even with the same ligands, the selectivity between different supports are identical. Once move one media to another, adsorption and elution conditions need to modify for achievement of similar results.
Salt Concentration
The addition of structured salts in equilibration buffer and sample can promote ligand-protein interactions in HIC. However, as salt concentration increases, the amount of bound proteins increases, and the risk of protein precipitation increases at the high ionic strength.
pH Range
The HIC mobile phases are normally in the neutral pH range from 5 to 7 with buffers of sodium or potassium phosphate. The interaction strength between proteins and media will decrease with pH increasing, this effect varies from different protein types. Therefore, pH can impact the protein binding levels and medial selectivity, but no significant effect over moderate ranges.
Temperature
The hydrophobic affinity of interaction increases with temperature, it also can impact protein structure, solubility, interaction with HIC matrix. Since the temperature effects are difficult to predict, it is not utilized to modulate separation in HIC.
Call Us
+86(021)-50795728
+86(027)-60707970
