Ion Exchange Chromatography Purification
QYAOBIO provides ion exchange chromatography for customers in worldwide
Ion exchange Chromatography (IEX) can separate proteins with different charge on surface, this results in high-resolution separation with high sample loading capacity. The IEX separations have basic of the reversible interaction between charged proteins and oppositely charged chromatography resins. Since the binding kinetics for IEX are fast with rigid chromatography particles, the ion exchange chromatography resin can be applied at high flow rates.
Mechanism of Ion Exchange
Normally, the net surface charge of proteins vary according to the surrounding pH. The pH where a protein has no net charge is isoelectric point (pl). Above the isoelectric point (pl), the protein can bind to the positively charged anion exchanger. Below the isoelectric point (pl), the protein can bind to the negatively charged cation exchanger.
Proteins can load onto the column at low ionic strength, then alter the conditions and make the bound substances desorbed differentially. The final elution is usually achieved by increasing salt concentration or changing pH gradient.
IEX Purification Steps
Ion exchange chromatography is the most popular chromatographic mode in bio-purification, it is widely applied downstream processing platforms. The typical scheme of IEX is applied in intermediate purification steps. Anion exchange chromatography is the integral part in most plasma protein purification platforms.
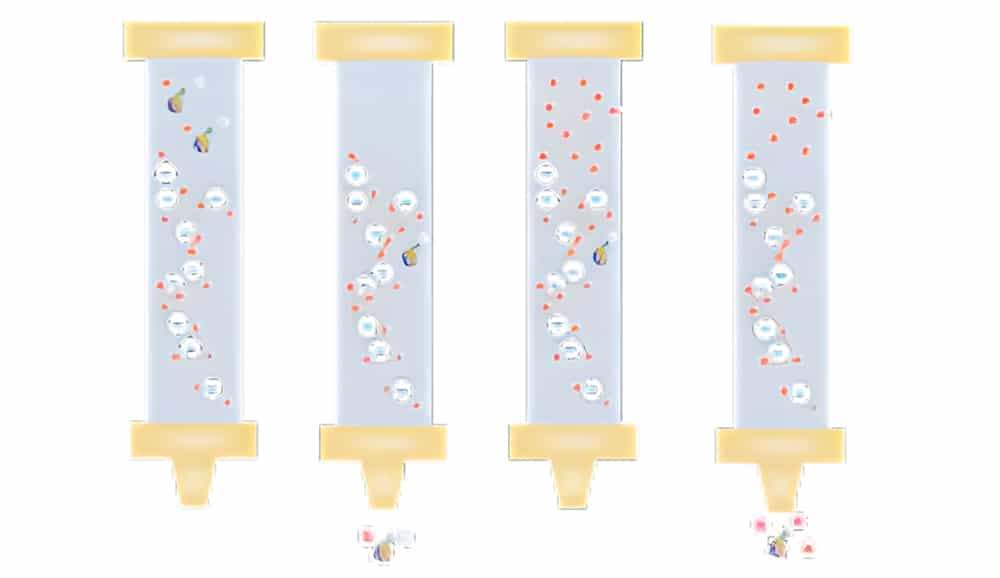
Equilibration
The equilibration of the stationary phase is the first step of IEX purification. Once reach the equilibration, all stationary phase charged groups are binding with exchangeable counter-ions, like chloride or sodium. Normally, we need to select start buffers with suitable pH and ionic strength, on order to ensure the target proteins binding to the medium.
Sample Application&Wash
Binding the target molecules and wash out all unbound components.
Elution
As the ionic strength increases in the column, the proteins with the lowest net charge are firstly eluted. Similarity, the proteins with the highest net charge are most strongly retained and eluted lastly.
Regeneration
The final washing with high ionic strength buffers will remove any bound molecules and regenerate the column. Then the column is re-equilibrated in start buffers, and start the next run.
Matrix in Ion Exchange
In ion exchangers, the insoluble matrix can bind to charged groups covalently. Then the charged groups are associated with mobile counter-ions, these counter-ions can exchange reversibly with other same charge ions without altering the matrix. It is possible to have both positively and negatively charged exchangers.
The matrix has base of inorganic compounds, synthetic resins, poly-saccharides. The characteristics of matrix determine the final chromatographic properties, like efficiency, capacity, recovery, chemical stability, mechanical strength, flow properties. The nature of matrix will affect the final behavior towards biological substance and the maintenance of biological activity.
The charged groups are the fundamental property of ion-exchangers. The type of groups determine the type and strength of the ion exchangers. There are a variety of groups in following table:
| Anion exchangers | Functional group |
| Diethylaminoethyl (DEAE) | -O-CH2-CH2-N+H(CH2CH3)2 |
| Quaternary aminoethyl (QAE) | -O-CH2-CH2-N+(C2H5)2-CH2-CHOH-CH3 |
| Quaternary ammonium (Q) | -O-CH2-CHOH-CH2-O-CH2-CHOH-CH2-N+(CH3)3 |
| Cation exchangers | Functional group |
| Carboxymethyl (CM) | O-CH2-COO |
| Sulphopropyl (SP) | -O-CH2-CHOH-CH2-O-CH2-CH2-CH2SO3 |
| Methyl sulphonate (S) | -O-CH2-CHOH-CH2-O-CH2-CHOH-CH2SO3 |
The sulphonic and quaternary amino groups can form strong ion exchangers, while other groups form weak ion exchangers. The terms of strong and weak refer to the variation extent of ionization with pH. Strong ion exchangers can ionize completely over a wide pH range, while weak ion exchangers in the narrow range.
The core properties of strong ion exchangers:
- The loading capacity never decrease at high or low pH values.
- Simple mechanism of interaction between the ion exchanger and solution.
- More controllable in ion exchange experiments, due to the charge characteristics of media will never change in different pH.
Application of Ion Exchange
It is difficult to separate similar proteins with little differences in molecular weight, or without tags, specifically in techniques of affinity or size exclusion chromatography. Therefore, the distinct physico-chemical properties of protein molecules are applied for further separation. As protein molecules have overall electrical charges, ion exchange chromatography (IEX) can separate positive or negative charge molecules by interaction with charged-ion exchange resins as stationary media.

The charged proteins can bind to the resins in normal buffering conditions, then be eluted in the mobile phase by increasing salt concentration, or changing of pH. According to the protein isoelecric point (pl) value, we can apply different cation or anion exchange chromatography media. Once the environmental pH is lower than pl, the protein will carry positive charge and bind to the cation exchange resins strongly. While proteins with negative surface charge bind to the anion exchange counterpart.
Call Us
+86(021)-50795728
+86(027)-60707970
