Stapled Peptides for Drug Improvement
Introduction
Stapled peptides are the new technology to bypass the intrinsic problem of peptide-drugs, this new strategy will increase the cellular uptake. Stapled peptides is consisting of peptide chains with α-helical structure by the external brace. The cross-link is achieved by the linkage of side chains with opportune-modified amino acids.
Peptides
Proteins have the pivotal role in different aspects of the cellular systems, from structural stabilization to nutrients transport, from immune defence to enzymatic activities. In recent years, the medical chemists apply the proteins as drugs for the various disease treatment, from metabolic disease to haematological pathologies, even to cancer treatment.
In fact, short peptide chains also play the crucial rules in the organisms as messengers for endocrine signals. This key role are exploited to obtain drugs as agonists in the signal transduction processes. Peptide drugs have some advantages in pharmacological field:
- Fast systemic absorption to avoid the first-pass metabolism
- Precise control in targeting
- Easily structural modifications for pharma-cokinetic
However, peptides as drugs also have some challenges, due to the natural characteristics:
- Unstable in cellular environment
- Easily hydrolyzed in acidic or enzymatic conditions
- More difficult to access to cell environments due to high molecular weight
- Hard to obtain large quantities due to numerous synthetic steps
Peptide-based drugs are easier to functionalize than other pharmaceuticals, this provides excellent chances to alter properties and achieve high target-selective. In addition, peptide drugs have low immunogenicity level, non-significant hepatic metabolism, and low level of drug-drug toxicology.
Stapled Peptide
The side chains in peptides, can be modified to alter peptide properties, especially proteolytic stability and solubility. The most promising technique to stabilize peptides is cyclization of linear peptides, the method especially for helical peptide is peptide stapling. This conformation can hide the amide bond inside the helix, enhance the protease stability, and increase the permeation of the cellular membrane.
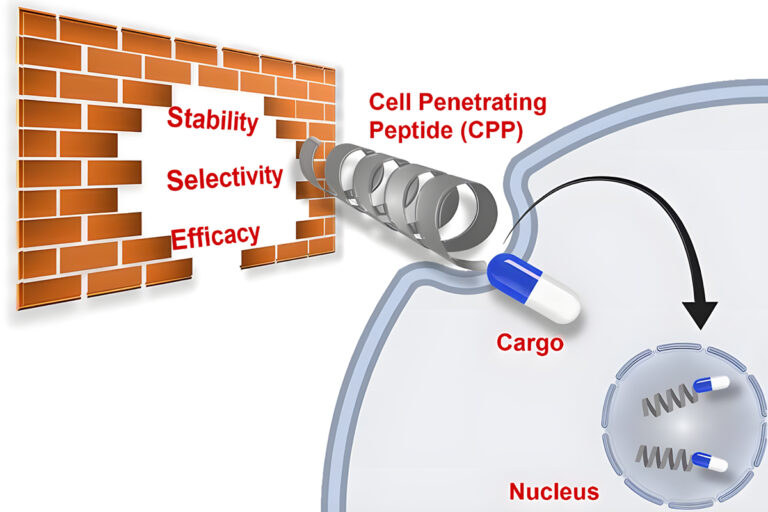
The high hydrophobicity of helical structure result in the biological cross of membranes. In fact, the α-helical moiety is essential for lots of trans-membrane proteins. Therefore, artificial helical peptides are the primary objectives of new peptide-based drugs. This kind of peptides can reach to a wide range of targets, without limitation of the external environments.
Stapled Peptide Properties
Protease Resistance
Stapled peptides have the most interesting properties of protease resistance. The helical structure can hide the protease targets and peptide bonds, in order to prevent the attack of the protease. The protease resistance correlates with the number of stapling bridges and the stabilization degree of α-helical.
In addition, stapled peptides also have excellent resistance in acidic environments. The insertion of stapling braces can enhance the peptide half-life, this property permits the oral delivery route of peptides.
Cellular Uptake
The major limitation of peptide-base drugs is the difficulty in penetration of cellular membranes. Because the cell-penetration is dependent on the hydrophobicity and helicity of peptides, stapling peptides can enhance the cellular uptake of peptides.
Experimental Determination
It is necessary to evaluate the properties of stapled peptides, like helicity, proteolytic resistance, cellular uptake, activity. In order to achieve the potential application by fundamental tests.
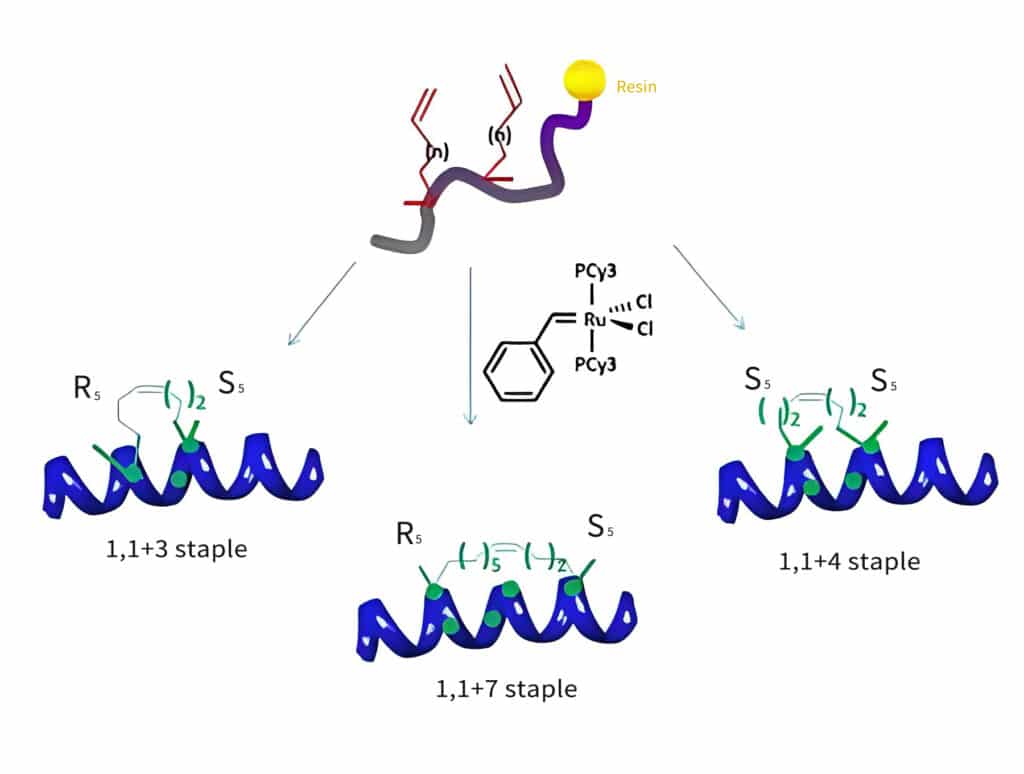
Peptide Stapling Technology
In peptide stapling, there are several different reactions to link the side chains in the same peptide. Therefore, mold reactions are involved without interfering with other functional groups, and maintaining unchanged chiral centre of each amino acids. The main principal stapling techniques are as following:
Ring Closing Metathesis
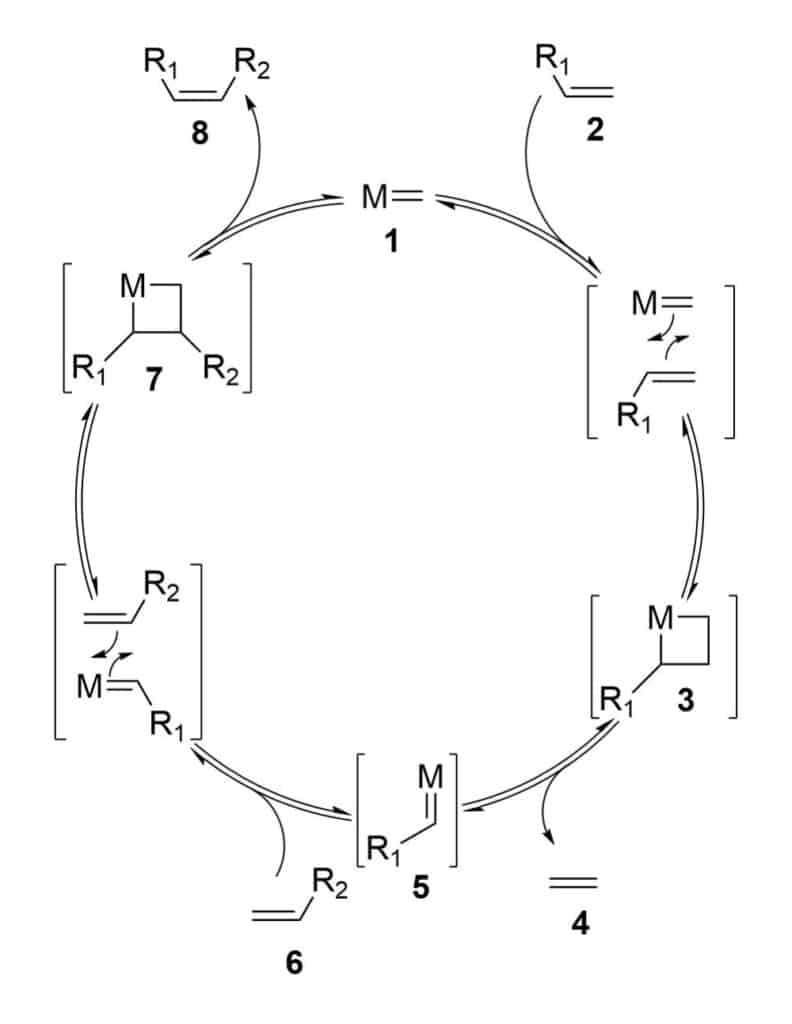
Ring closing metathesis is the most promising reaction in peptide stapling, this reaction is the coupling between two terminal alkenes, this results in macrocycle formation by a double bond with loss of an ethylene molecule.
The ring closing metathesis usually provides both E- and Z-isomers. It is difficult to separate and assign these two isomers. Therefore, it is possible to reduce the double bond moiety, and obtain the full-saturated bridge.

This reaction mechanism is on the base of double cycloaddition/cycloelimination between an olefin and the carbene-metal complex.
Modified Peptide Methods
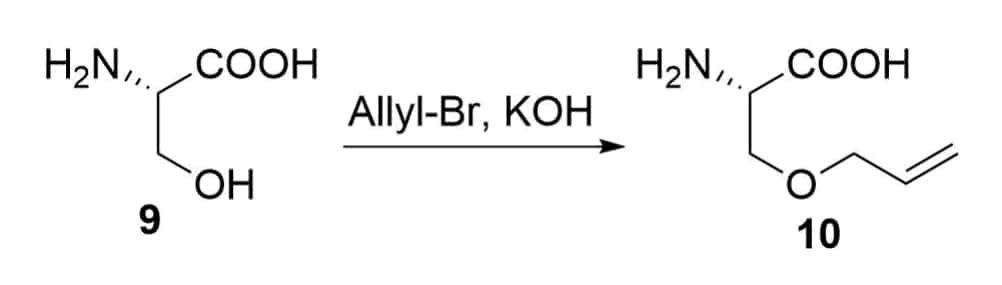
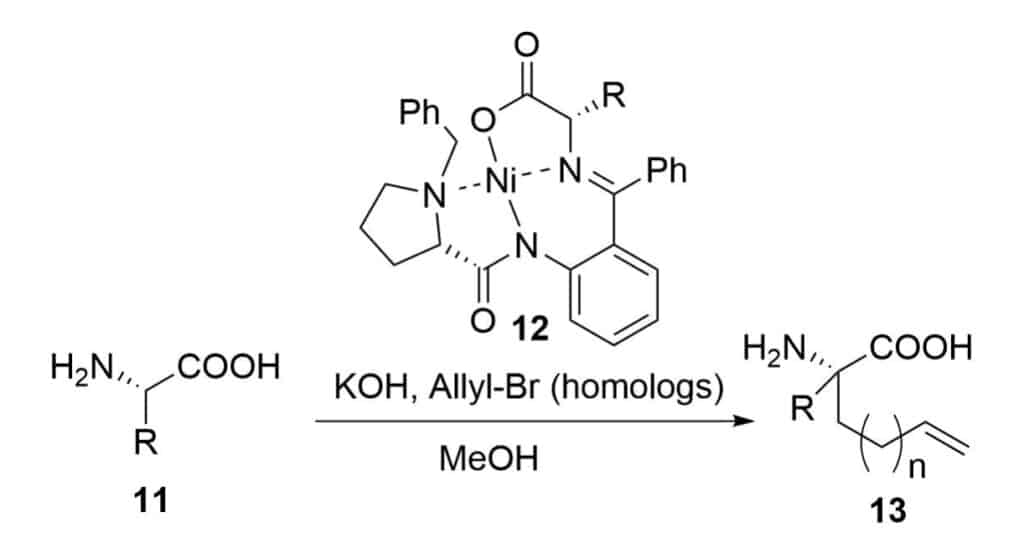
It is necessary to have two olefinic-substituted amino acids in the formation of ring closing metathesis. There are two ways to introduce the olefinic moiety on an amino acid.
The allylation of the serine by the nucleophilic substitution reaction
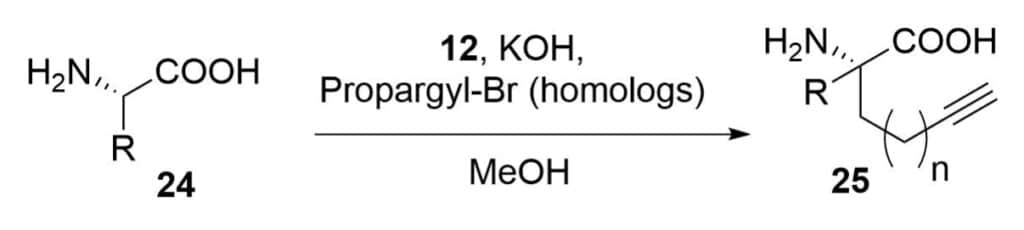
The chiral nickel catalyst substitution reaction
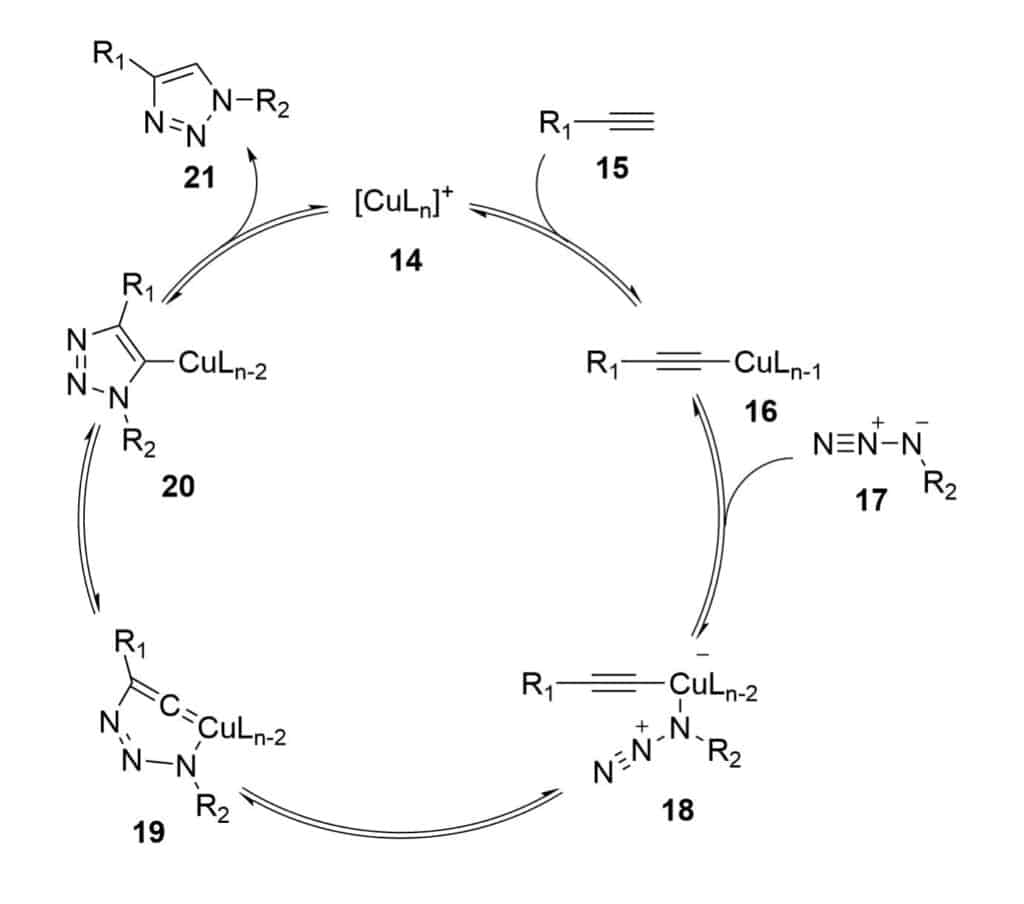
Copper Catalyzed Azide Alkyne Cycloaddition
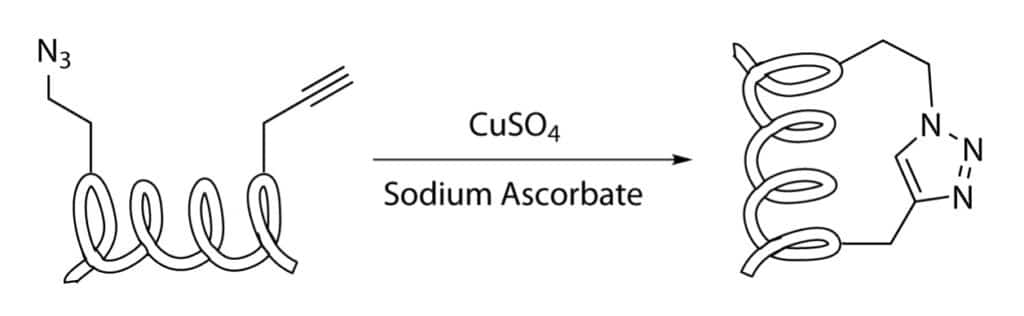
CuAAC ( copper catalysed azide-alkyne cycloaddition) is also known as click-chemistry, Huisgen cycloaddition. It is a useful tool for peptide stapling, this reaction consists of regioselective-cycloaddition between an azide and a terminal alkyne, and forms a 1,4-disubstituted 1,2,3-triazole ring.
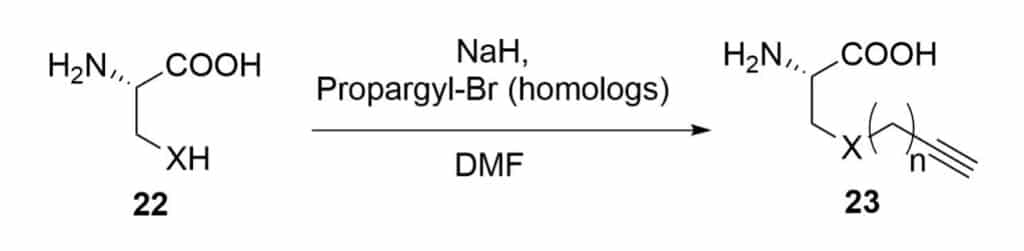
Modified Peptide Methods
Nucleophilic substitution on propargyl bromide
Ni-catalyzed Double-bond substituted reaction
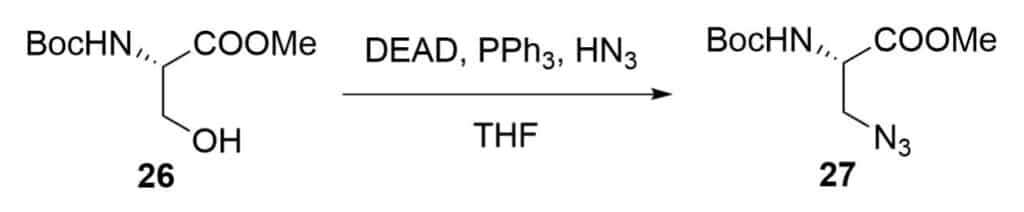
Azide-amino acid in Mitsunobu reaction
Mesylation of the Weinred amide
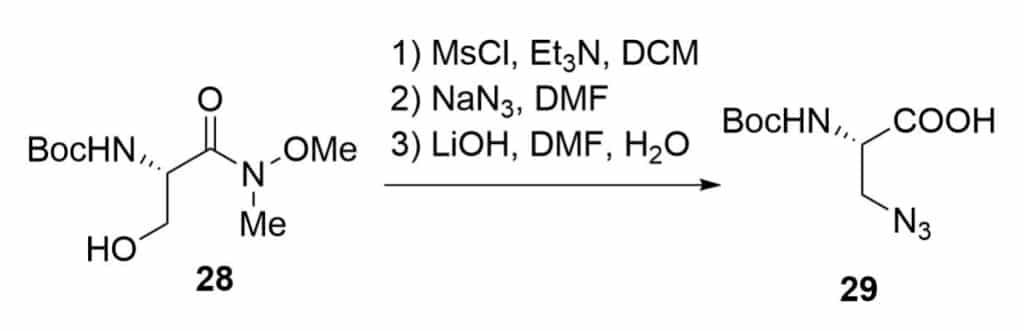
Hoffmann rearrangement
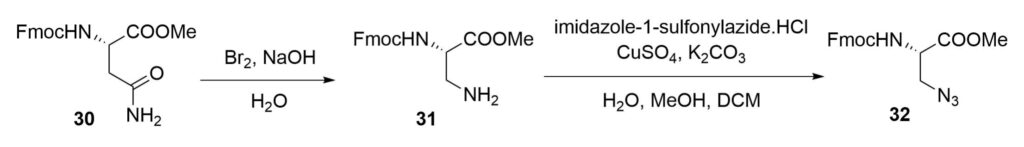
Ullmann-type coupling
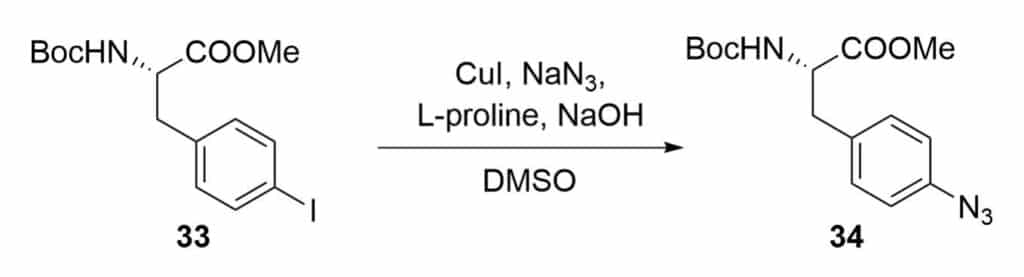
Lactamization Reaction
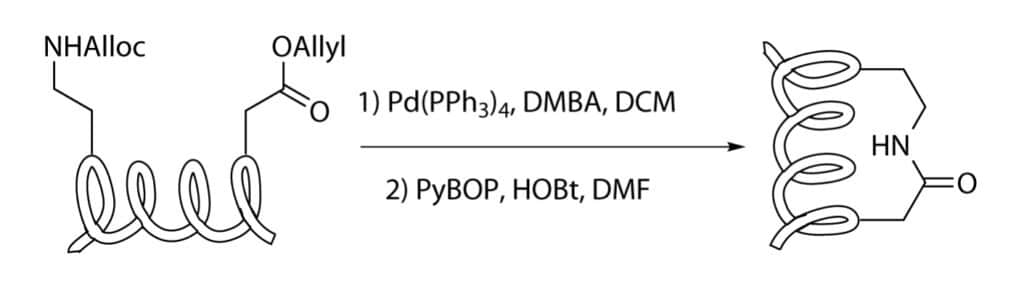
Amidic bond formation is another way to form stapled peptides, The lactamization reaction is a classical amide formation. The common reaction mechanism of lactamization condensation is as following:
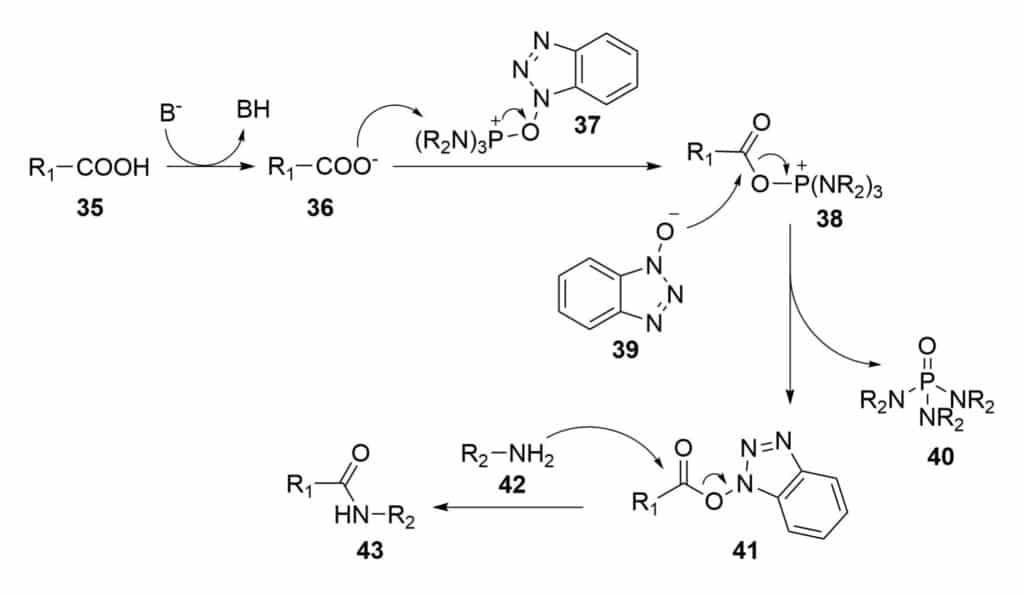
It is necessary to protect the functional groups in stapling process, in order to avoid undesired coupling with the functionalities.
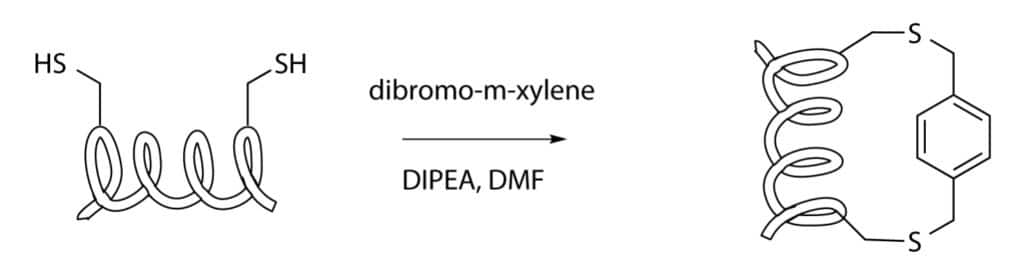
Cysteine-Xylene Stapling
Cyteine-xylene stapling apply the double nucleophilic substitution on an α,α’-dibromo-m-xylene of two cysteine in side chains. This stapling provides the stabilization of disulphide bridge, the insertion of xylene crosslinker will contribute to the bridge stabilization, enhance the spacer wideness.
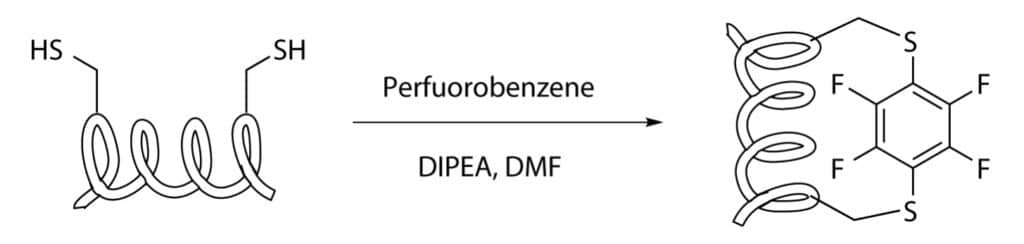
Cysteine-Perfluorobenzene Stapling
Cysteine-Perfluorobenzene Stapling is similar to cyteine-xylene stapling, this reaction is an aromatic nuclephilic substitution with a hexafluorobenzene substrate.
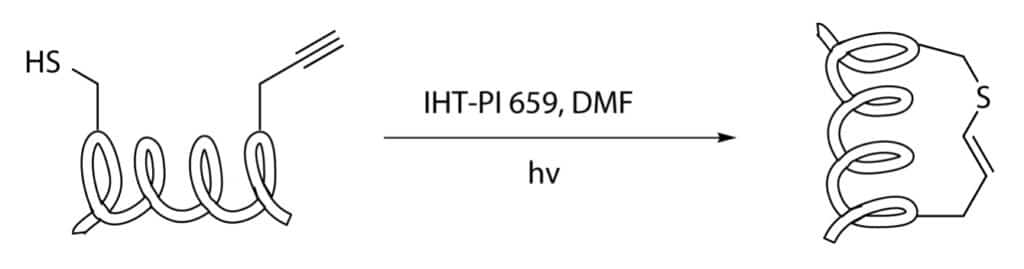
Thiol-yne/-ene Click Chemistry
Thiol-yne/-ene stapling applies an anti-Markovnikov way to couple a cysteine residue with a terminal alkyne-substituted amino acid.
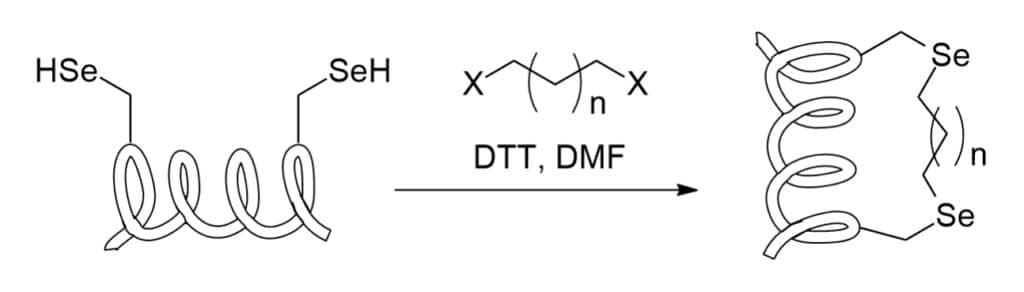
Selenocysteine Stapling
Selenoysteine can be applied in synthesis of stapled peptides, the double electrophile is the linker between two selenium atoms.
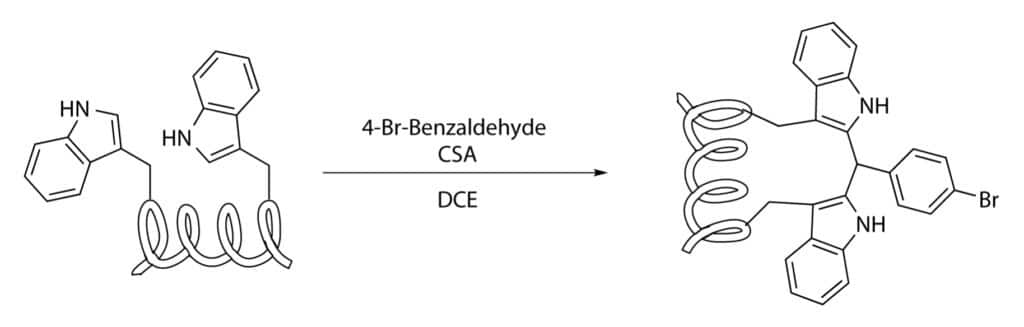
Tryptophan Condensation
Tryptophan residues are employed to staple peptides, these amino acids contain an electron rich aromatic heterocycle. It can couple with iodophenylalanine or iodotyrosine by palladium-catalysed C-H activation.
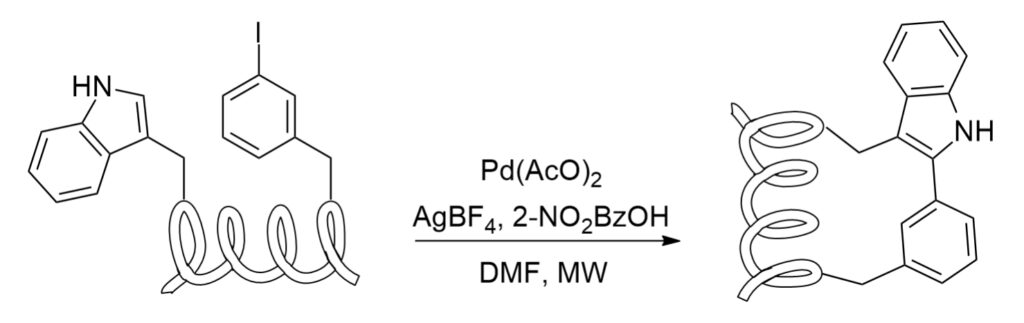
C-H Activation
C-H activation is a useful method in synthesis of tryptophan-containing peptides, the metal catalysis can promote the coupling between the C-2 of the indolic core and the side chain of iodophenilalanine or iodotyrosine.
Conclusion
There are different factors determining the final properties of stapled peptides, such as: stapling position, peptide nature, length of staple brace. Therefore, peptide stapling is a efficient way to bypass the main problems of peptide drugs. The forced α-helical conformation enhances the hydrophobicity, increase the cellular uptake, and increase the protease resistance.