Synthesis of Multi Antigenic Peptides
Introduction
Multiple antigen peptides (MAPs) are a kind of polymeric peptide macromolecules bearing radial branches. It is a highly effective method for producing high-titer anti-peptide antibodies and synthetic peptide vaccines. MAP is essentially composed of three structural elements: (1) an amino acid, like glycine or f-alanine, that is bonded to a resin, (2) an inner core matrix that multimerizes peptides and allows the MAP arranged in cascade, (3) a surface layer of peptide antigens attached to the inner core matrix. Since Lys has two ends, α- and ɛ-amino groups, which can be used in branching reactions, it is the most often employed amino acid in the core matrix.
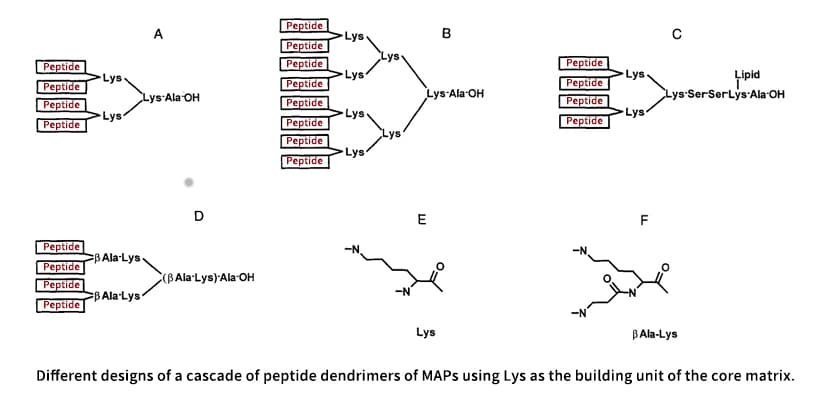
It is an advantage that MAP has an immunological focus due to it consists of nearly pure antigens. In an octameric MAP of a 15-mer peptide, the peptide antigen would make up more than 90% of its weight, but the branching Lys core is small, less than 10% of its weight and immunologically silent. In contrast, conjugated peptide antigens, which make up less than 20% of the weight of conventional, big, immunologically active protein carriers, are dispersed randomly across the protein carrier. As a result, the inefficiency of linear peptides in producing antibodies can be overcome by multimerization in the MAP design.
Stepwise Synthesis of MAPs
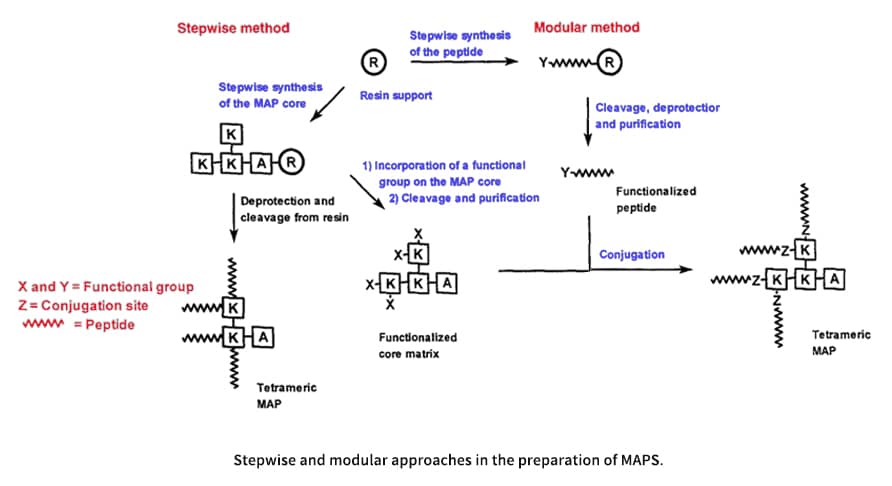
There are two general methods (direct and indirect) to prepare MAPs. The majority of syntheses are accomplished by direct methods using stepwise procedures to prepare MAPs sequentially, which is practically similar to a single-chain peptide was synthesized on solid supports. The purification of MAPs requires considerable effort because they are macromolecules and side products produced during the assemblage step are amplified, resulting in microheterogenicity. Based on the numerous successful applications, it seems that heterogenicity has little bearing on the majority of immunizations. However, chemically unambiguous MAPs are preferred for clinical or other nonimmunological purposes.
Stepwise Synthesis
The stepwise preparation of MAPs is a repeated, continuous process that is easy to use. To achieve the desired level of branching, this approach starts with a C-terminus with a small amino acid, and then the core is constructed via diprotected Boc-Lys(Boc) in Boc chemistry or Fmoc-Lys(Fmoc) in Fmoc chemistry. Finally, the selected peptide antigen is sequentially extended to the lysyl core matrix on the resin, allowing the desired MAPs to be formed. Peptide antigens with a C→N orientation are generated using this stepwise method.
Homomeric MAPs
Fmoc chemistry is base-driven, in contrast to Boc chemistry, which is acid-driven. In Fmoc chemistry, the release of side chain protecting groups and resin supporting bonds does not require the use of very strong acids. Base-driven chemistry has the advantage of eliminating the requirement for the neutralization phase and simplifying the repetitive cycles. Additionally, the peptide linkage to the resin support are cleaved only needs a mild acid like TFA for Fmoc-tert-butyl chemistry, whereas the Boc-benzyl chemistry requires HF or TFMSA. For laboratories that are not equipped or do not have access to a HF apparatus, the use of Fmoc chemistry procedures is undoubtedly the most appropriate.
Heteromic Synthesis of Chimeric MAPs
There are two methods for synthesizing chimeric MAPs bearing two different types of peptides: a simple method is to link them tandemly in a continuous array, and synthesizing them on the different arms of the core matrix is a more difficult method, which can be accomplished via a core matrix containing two different, orthogonally protected amino groups such as Boc-Fmoc, Fmoc-Aloc, Fmoc-Dde and Npys-Fmoc. Utilizing combinations of these protecting groups, the diepitope MAP can be obtained by stepwise synthesis of Lipidated MAPs in either arm. There have been several suggested techniques for diepitope MAPS, including fragment condensation and ligation.
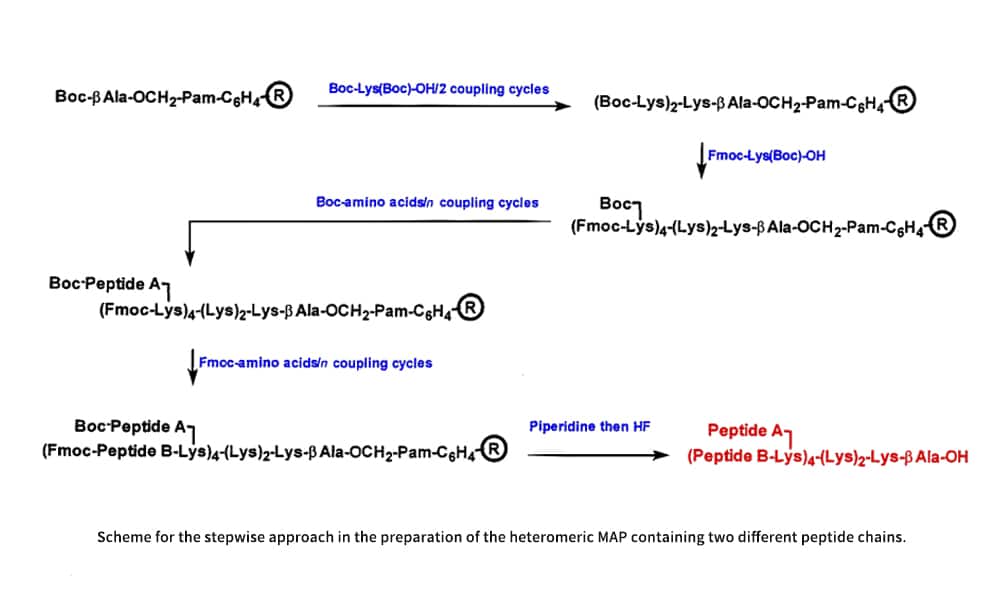
Lipidated MAPs
Lipidated MAP was produced by introducing a lipophilic moiety to the carboxyl terminus of MAP as an improvement toward the production of better synthetic peptide immunogens. The lipophilic moiety also has the ability to anchor the lipid matrix and act as a built-in adjuvant. There have been employed two kinds of lipophilic moieties: single lipid chain and clustered lipid chain that is anchored by a Cys such as a tripalmitoyl-derivatized Cys, tripalmitoyl-S- Glyceryl-Cys(P3C). On the COOH terminus of a MAP, both kinds of lipids are subsequently linked to the side chain of one or more Lys residues.
Multiple Cyclic Antigen Peptides
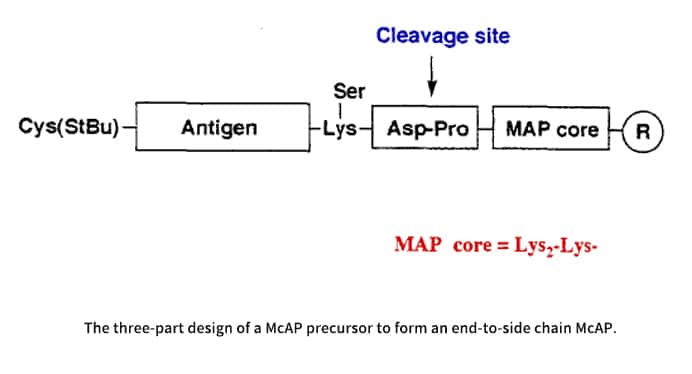
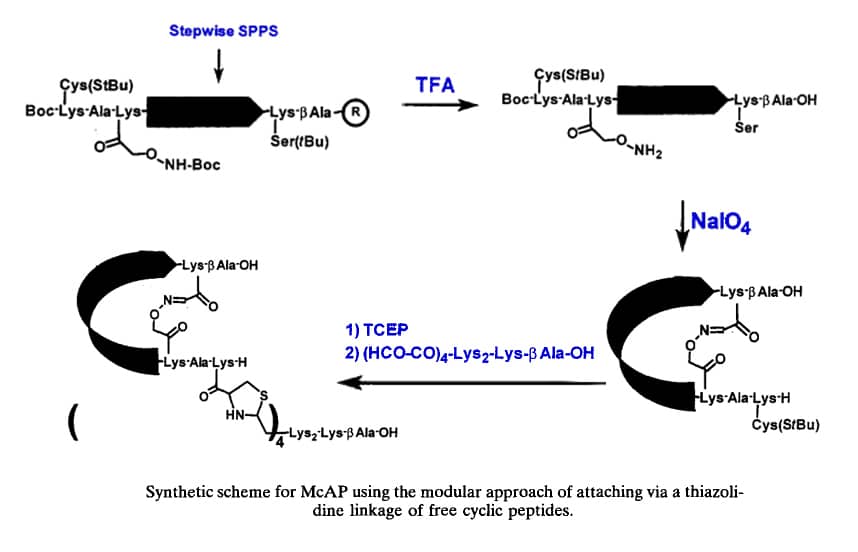
Constrained peptides, such as the multiple cyclic antigenic peptides (McAPs), are highly desirable to mimic peptide-derived conformations. The design of McAPs is tripartite. Common regions comprise the first two sections. Part 1, the MAP core matrix, has a tetralysyl architecture and a short peptide. Part 2 has a particular chemical cleavage site, which provides a monomeric cyclic peptide to confirm the intrachain cyclization yield. If verification is not required, this section may not be necessary. Part 3 is the variable region that includes a peptide antigen framed by a Lys at the COOH terminus and a Cys(StBu) at the amino terminus. In order to facilitate end-to-side chain cyclization via thiazolidine formation with the Cys at the amino terminus, a masked aldehyde is connected to the side chain of the COOH-terminal Lys.
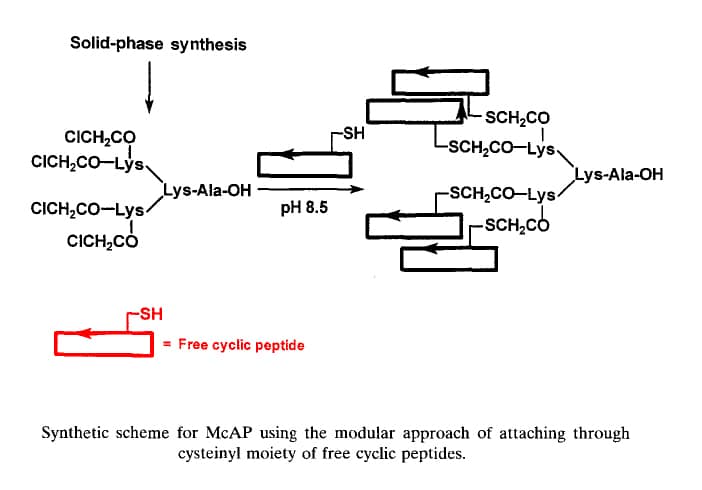
Modular Synthesis of MAPs
Orthogonal ligation is a method to obtain highly purified MAPs, in which purified unprotected peptide segments are joined to the core matrix. Modular synthesis by orthogonal ligation offers the following advantages: (1) Several types of epitopes can be flexibly combined to form di- or tri-epitope MAPs, and the orientation of their attachment to the core matrix can be selected. (2) MAP products can be easily purified using traditional procedures. In order to form MAPs with non-amide bonds, two methods have been developed to ligate unprotected peptides: general methods based on thiol and carbonyl chemistry. The need to generate amide bonds that can be used in the synthesis of peptide dendrimers led to the development of new ligation techniques. During solid-phase synthesis of thiol and carbonyl chemistry, the purified synthetic peptide monomer and the core matrix place, respectively, a reactive pair consisting of a nucleophile and an electrophile.
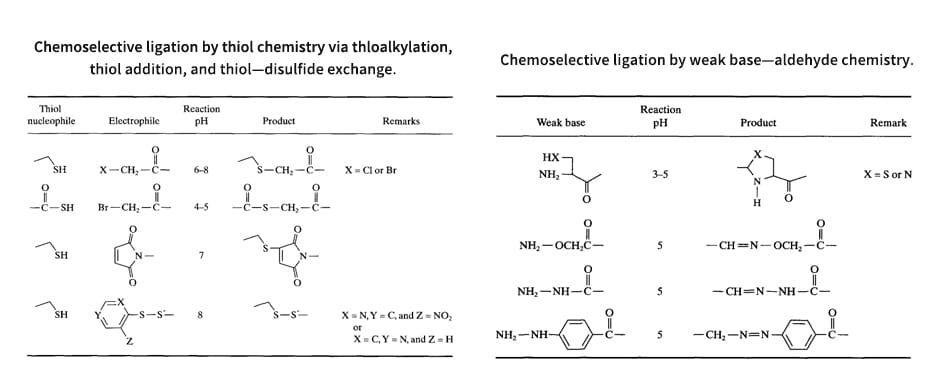
Thiol Chemistry
Thiol chemistry takes advantage of the selective reactivity that alkylation of sulfhydryl with halocarbonyl, sulfur-sulfur exchange with disulfide, and addition to conjugated olefins. The addition of thiol of Cys to an activated double bond of a maleimide group guides the formation of thioethers. An example is now provided to show the applicability of thiol chemistry: Free, end-to-end cyclic peptides are coupled to form McAPs by S- →N-Acyl transfer reaction and thioether formation.
Carbonyl Chemistry
Another approach along the same principles of thiol chemistry is to exploit the selectivity of other weak bases, particularly in the condensation reaction with aldehydes. When McAP is prepared via carbonyl chemistry, the constrained peptide is ligated to a MAP core matrix using weak base aldehyde chemistry. It is demonstrated that the versatility of carbonyl chemistry via thiazolidine, oxime, and hydrazone (hydrazide succinyl and 4-hydrazinobenzoyl group) linkages. For example, cyclic peptides are coupled through thiazolidine bonds to form McAP.
Conclusion
This article presents the use of different techniques to generate MAPs with one (homomeric) and two or more linear peptides (heteromeric MAPs) in different orientations, as well as MAPs containing cyclic structure (McAP, multiple cyclic antigen peptide). Qyaobio effectively employs these strategies to prepare MAP with high purity and good yield.