Unnatural Amino Acids
Unnatrual amino acids can be incorporated into custom peptides.
Unnatural amino acids are also known as non-proteinogenic amino acids, which are artificial synthesis or natural generation without incorporation into proteins and peptides. As unnatural amino acids never exit in proteins, enzymes cannot recognize them as cleavage sites for proteolytic degradation. Therefore, unnatrual amino acids can be incorporated into custom peptides for a variety of applications, such as conformation constraints, pharmacological active ingredients, diversely constructive combinations, robust molecular scaffolds. Unnatural amino acids can incorporate into custom peptides with appropriate protection groups.
Unnatural Amino Acid Functions
Unnatural or unusual amino acids are not naturally gene-encoded amino acids, these AAs are also known as non-proteinogenic amino acids. These special amino acids are chemically synthesized as pharmacological motifs, or naturally occur in plant or bacteria post-translation. These modified amino acids contribute to special bio-activity of enhanced peptide pharmacological activity and potency.
Modified peptides with unnatural amino acids perform functions as following:
- Improve selectivity of peptides
- Improve receptor binding
- Modify activity as agonists or antagonists
- Increase half-life in vivo
- Enhance penetration across cell membranes
Unnatural Amino Acids Classification
The multitude of unnatural amino acids are available in custom peptide synthesis. The incorporation of amino acids result in different characteristics of final peptides. Such as: affinity enhancement, selectivity and stability of peptide drugs, induction and stabilization of secondary structures (α-helices, β-sheets, β-turns). Therefore, these unnatural AAs are applied widely in combinatorial libraries or as chiral building blocks.
Qyaobio provides many unnatural amino acids in peptide incorporation. These non-natural amino acids including:
D-amino acids
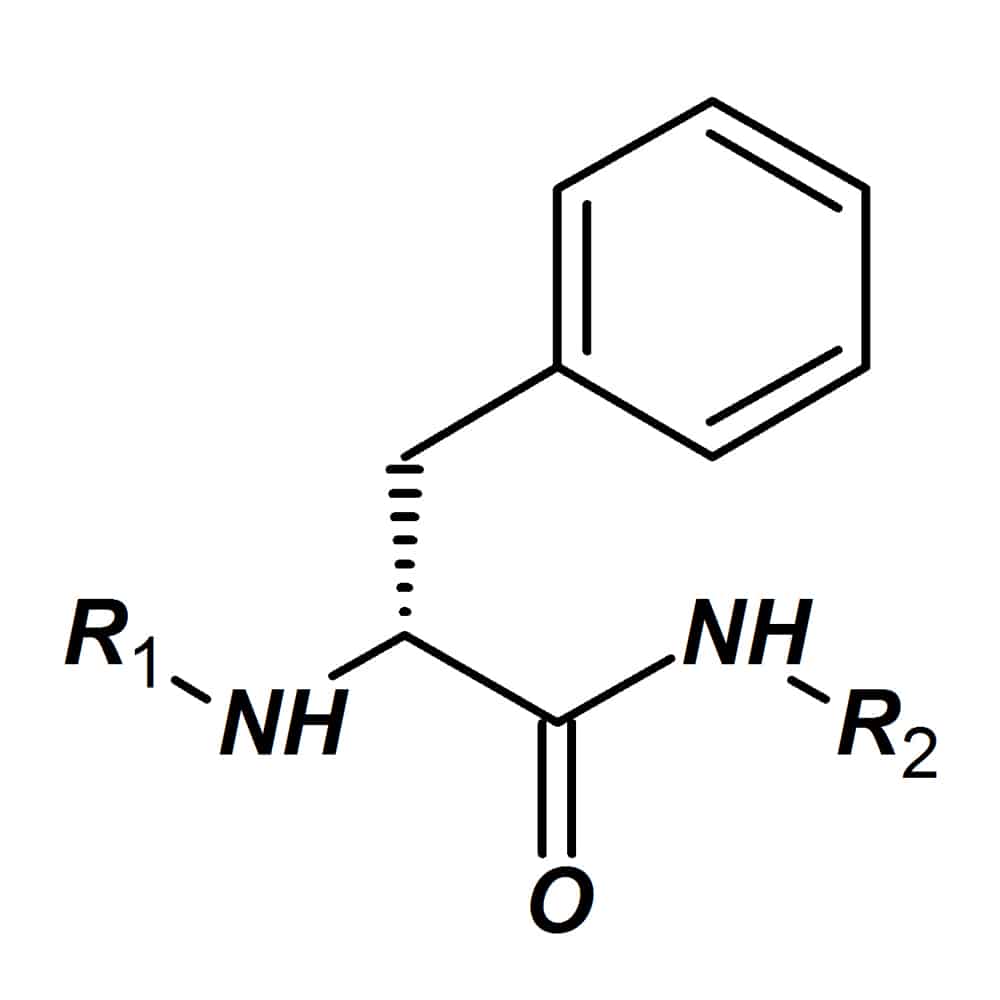
D-amino acids have the mirror image of natural L-amino acid isomers. These amino acids have wide applications due to the increased resistance against a range of degradation enzymes. Peptides containing D-amino acids are more stable than peptides with only L-amino acids. In some specific cases, peptides with D-amino acids also provide higher bio-activities than the natural L-variants
Homo-amino acids
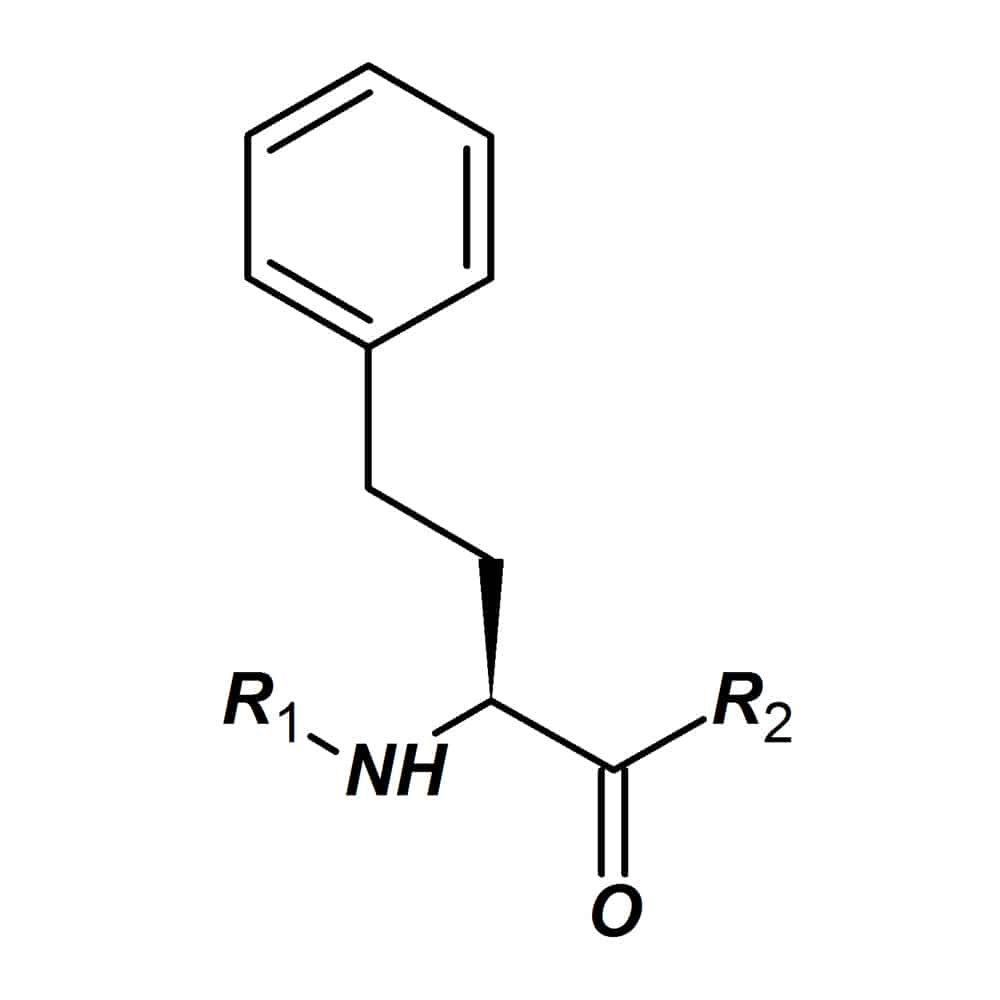
In homo-amino acids, the homo indicates the addition of a methylene (CH2) group on the α-carbon of an amino acid. These homo-amino acids are applied to create peptides with altered biological characteristics, like: enhanced biological activity, higher biological stability.
Beta-homo-amino acids
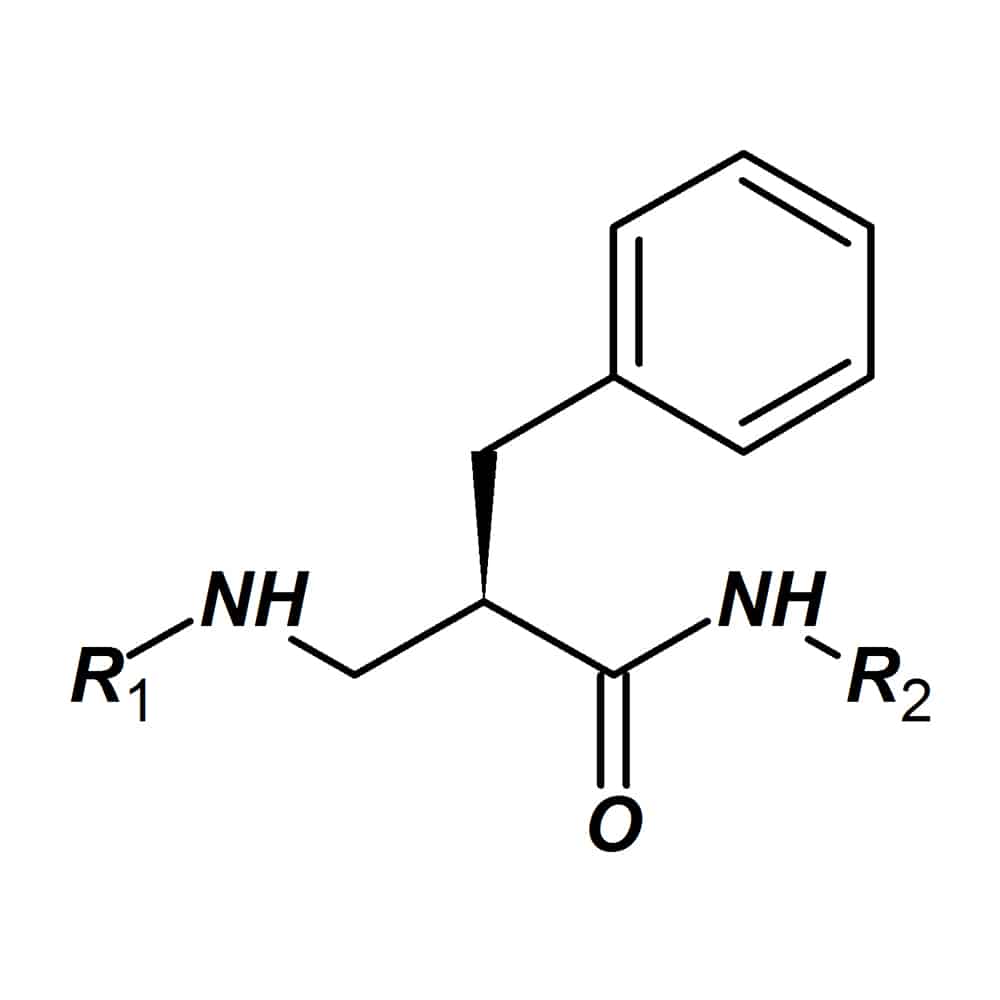
Beta-homo-amino acids (β-homo-amino acids) are analogs of stand amino acids, the carbon skeleton is prolonged by insertion of one carbon atom after the acid group. The peptides with β-homo-amino acids incorporation have improved pharmacological properties. The known effect of β-homo-amino acid incorporation is increasing half-life of peptide drugs in vivo. Beta-homo-amino acids also can increase potency, selectivity and non-toxicity in synthetic peptides.
N-methyl amino acids
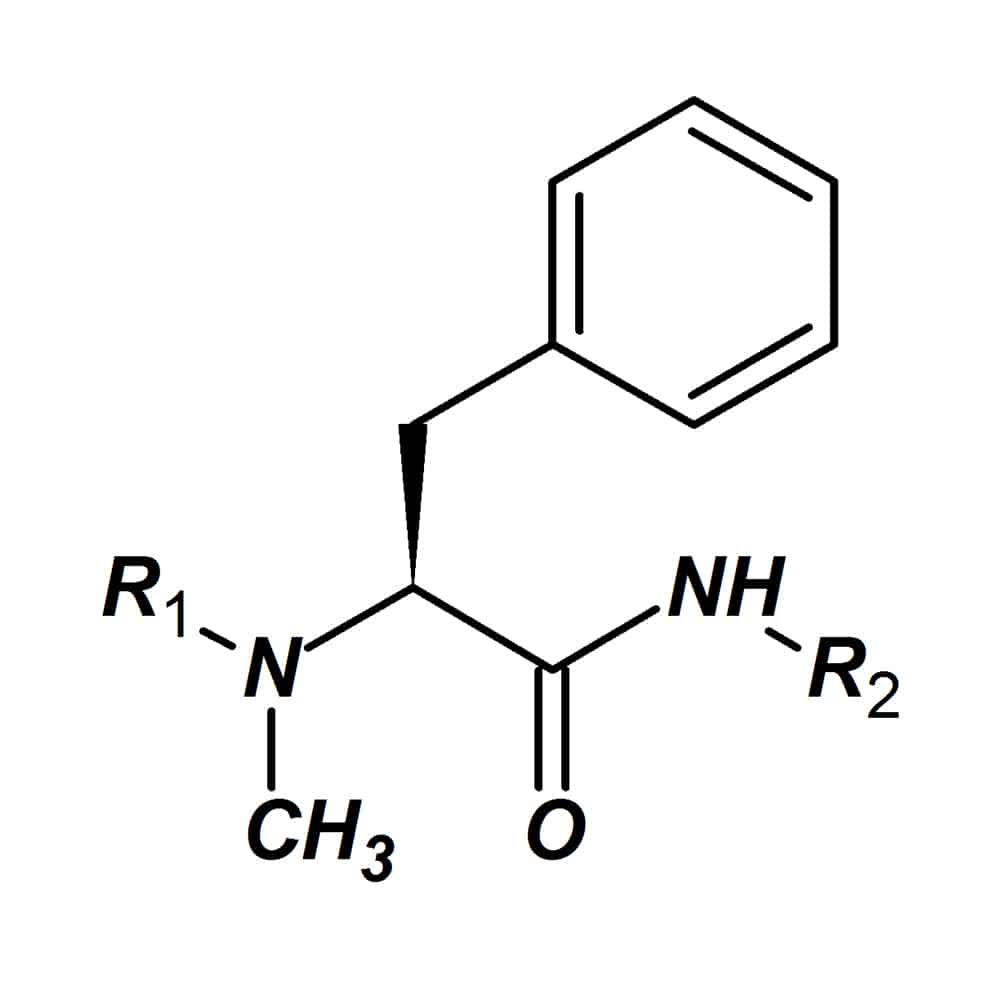
N-methyl amino acids carry a methyl group at the nitrogen instead of a hydro atom. Once replace the standard amino acids, N-methyl amino acids can improve the pharmaceutical properties of bio-active peptides. Introducing of N-methyl amino acids can increase the enzymatic stability of peptides, thus prolong the peptide biological half-life. In addition, substitution with N-methyl amino acids also can improve peptidase stability and enhance intestinal permeability.
Alpha-methyl amino acids
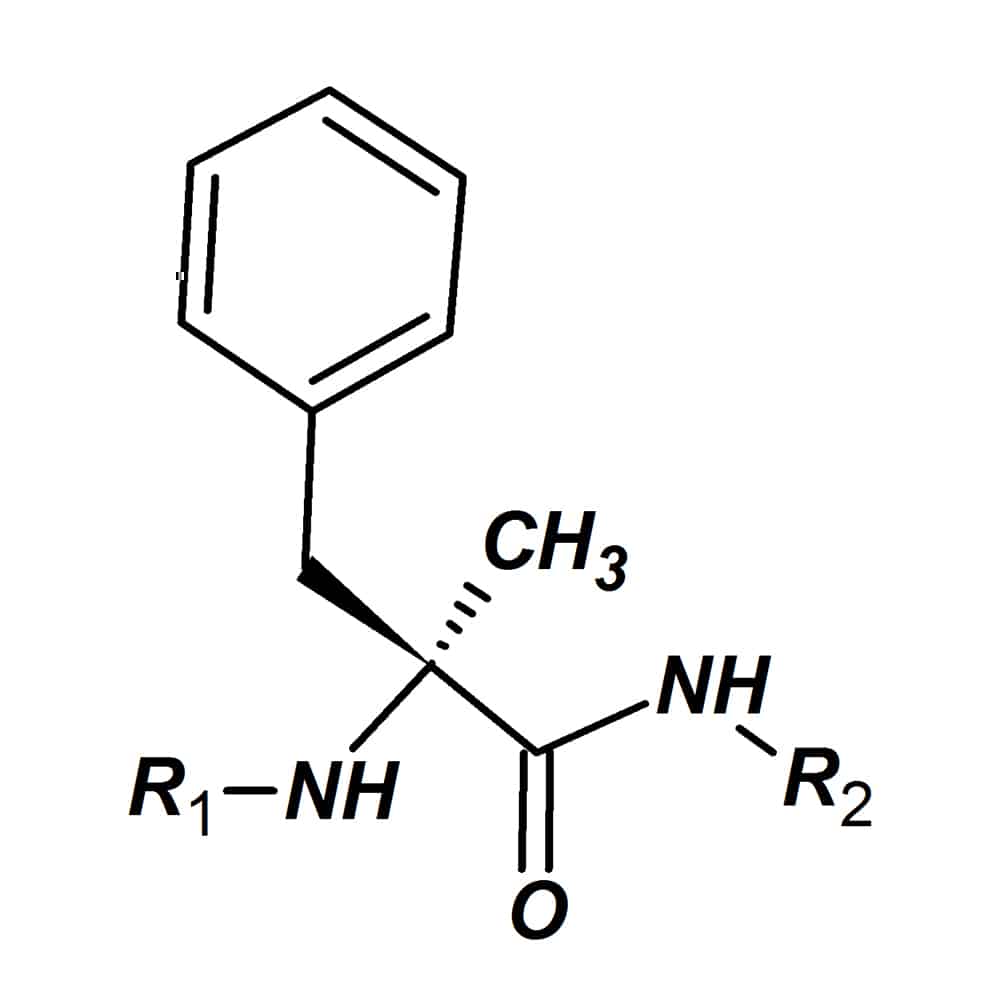
Alpha-methyl amino acids (α-methyl amino acids)are another variant type of natural amino acid, the hydro atom on the α-carbon is substituted by a methyl group. As a result, the α-methyl amino acids are totally stable in racemization/epimerizatio, since there is no possibility for abstracting the proton. Furthermore, alpha-methyl amino acids increase the proteolytic stability of custom peptides.
Unusual amino acids
Unusual amino acids are found frequently in microbial peptides and proteins with post-translation formation. The incorporation of unusual amino acids normally result in the special bio-activity of synthetic peptides. In peptide drug development, unusual amino acids are applied to enhance pharmaceutical properties of peptide drugs.
The prominent examples of unusual amino acids are:
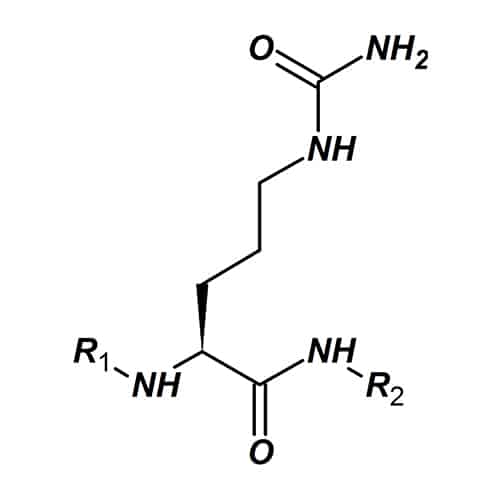
citrulline (Cit)
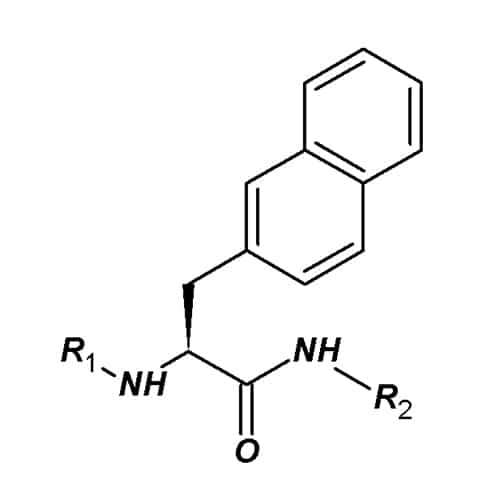
naphtylalanine (Nal)
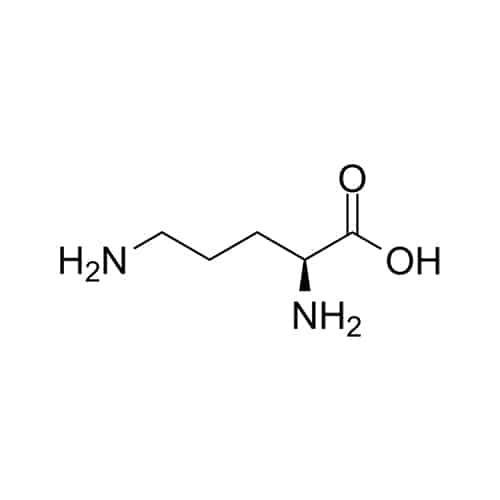
Ornithine(Orn)
norleucine (Nle)
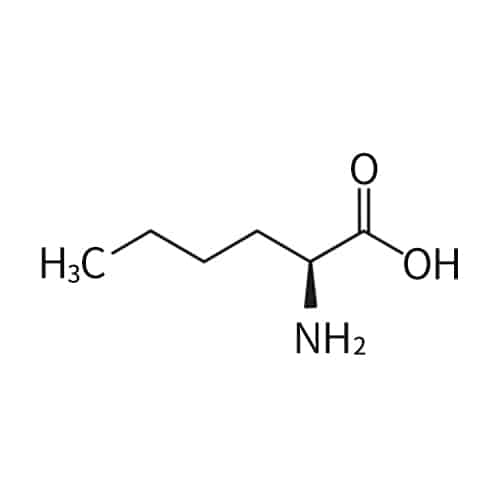
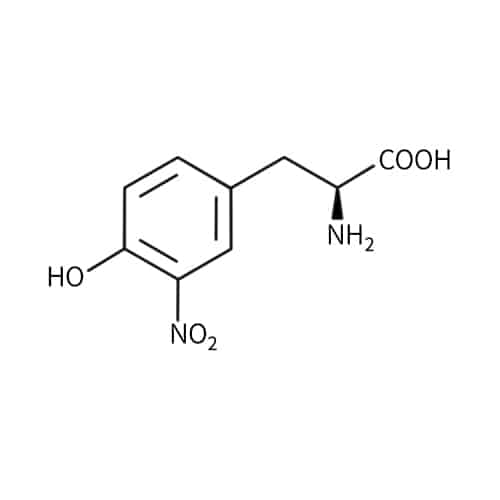
Nitrotyrosine(NT)
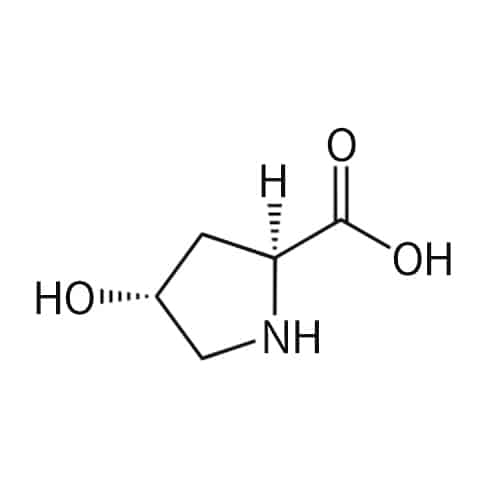
Hydroxyproline
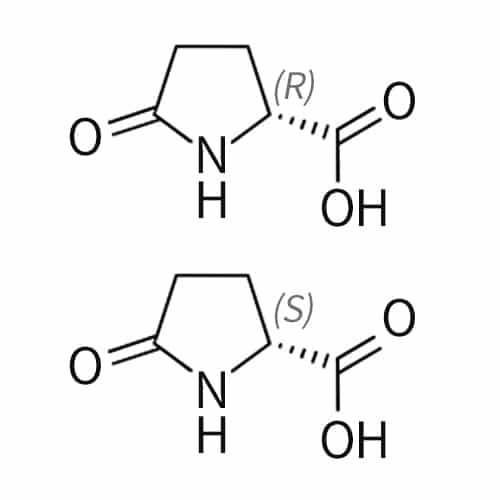
pyroglutamic acid(Pyr)
Abu (α-Aminobutyric acid)
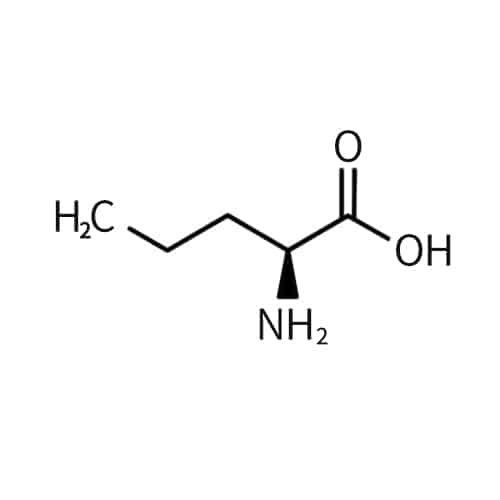
DAB (2,4-Diaminobutyric Acid)
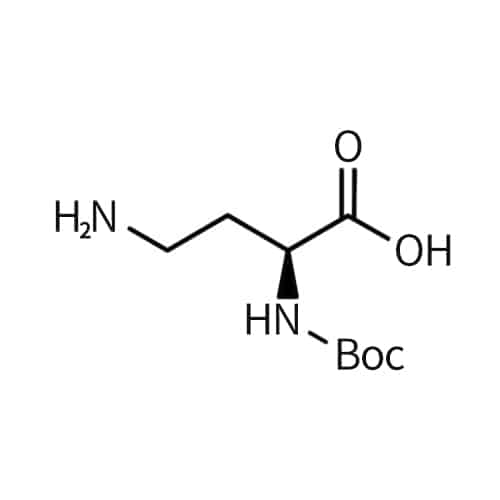
Nitroarginine
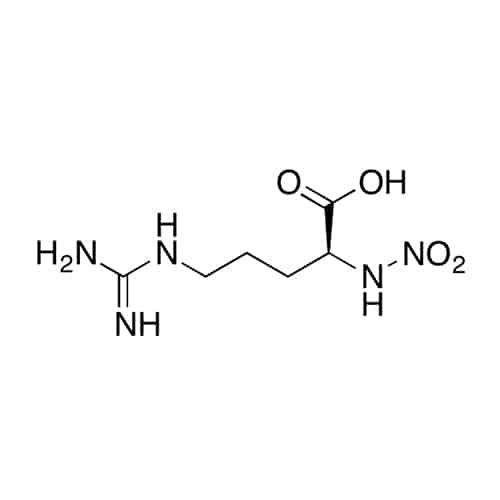
Methionine sulfoxide
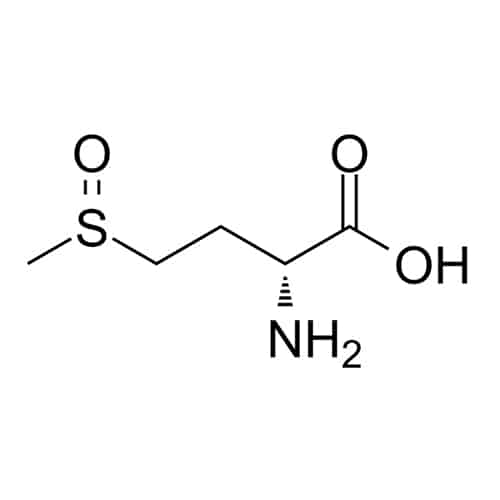
Beta-alanine
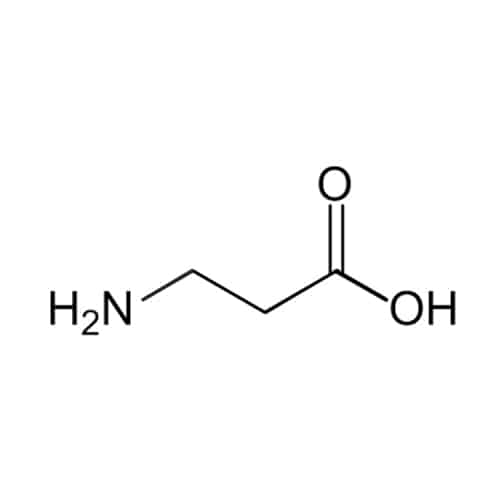
Regardless of the above unusual peptides, Qyaobio also provides other unusual amino acids, like helix/turn stabilizing motifs, backbone modifications of peptoids.
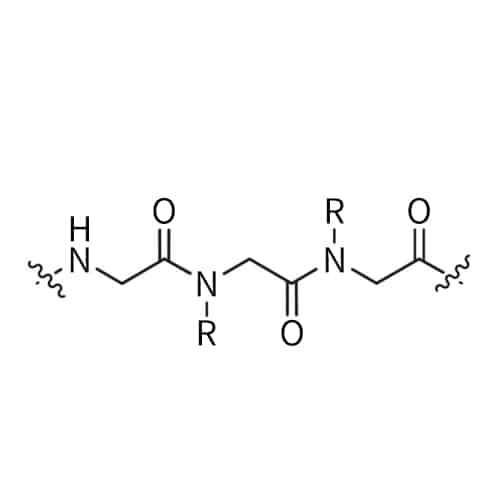
Peptoid
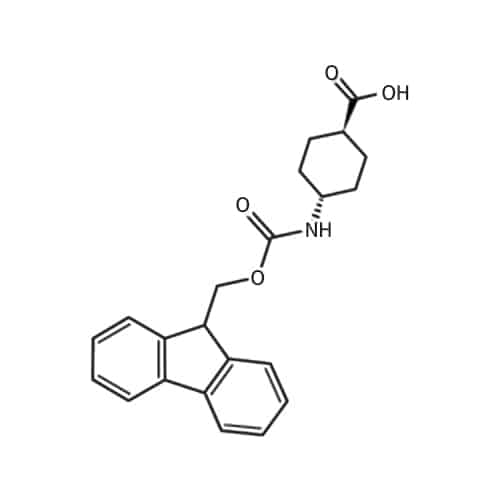
1,4-trans-ACHC-oh
Special Function UAAs
Special function UAAs are useful for specialized synthesis applications. In addition, PTM amino acids are available for starting materials for modified peptide synthesis. Post-Translational Modification (PTM) amino acids are also a new catalog of unusual AAs, these including different modification methods, including:
- Azide and Alkyne building blocks
- Acetylated amino acids
- Glycosylated amino acids
- Methylated amino acids
- Phosphorylated amino acids
- Stapled building blocks
- Dye labeled amino acids
- Pegylated amino acids & Special linkers
- N-α-Methyl Amino Acids
- Beta-Amino Acids
- Heterocyclic Amino Acids
Qyaobio is committed to provide a wide range of unnatural, non-stand amino acid peptide modifications. In order to satisfy the discovery and research requirement of peptide drugs.
Unnatural Amino Acid Analogs
Unnatural amino acids (UAADs) are exploited to enhance the stability and functionality of therapeutic targets. Our UAAD analogs including:
- Alanine, Glycine, Valine, and Leucine Analogs
- Arginine and Lysine Analogs
- Aspartic and Glutamic Acids Analogs
- Cysteine and Methionine Analogs
- Phenylalanine and Tyrosine Analogs
- Proline Analogs
- Serine, Threonine, and Statine Analogs
- Tryptophan Analogs
Call Us
+86(021)-50795728
+86(027)-60707970
